Perianal fistulising disease (PFD) is a complication that affects about 20% of patients with Crohn’s disease (CD) whose aetiology remains unknown. We aimed to identify predisposing events driving fistula formation.
DesignRectal biopsies from patients with CD with or without PFD (CD+PFD and CD, respectively; n=31) were collected and subjected to single-cell RNA sequencing. Functional analyses were conducted using peripheral CD3+ T cells, intestinal tissue explants, primary fibroblasts and two-dimensional epithelial monolayer cell cultures.
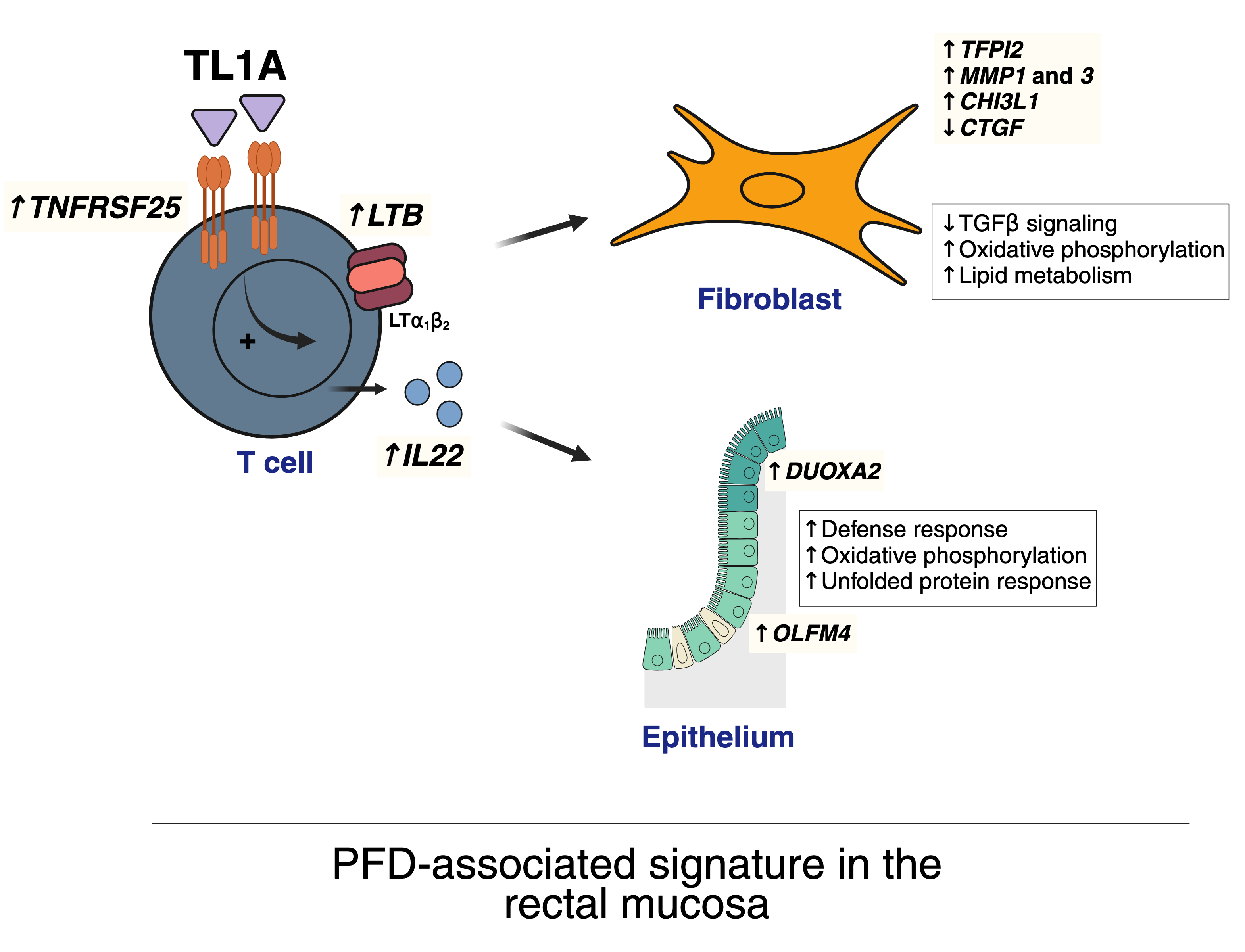
ResultsThe rectal mucosa of patients with CD+PFD is imprinted with cellular and transcriptomic alterations specific to PFD and independent of luminal inflammation, potentially driven by tumour necrosis factor-like ligand 1A (TL1A) activation in CD4+ T cells.
We identified lymphotoxin beta (LTB or its functional heterotrimer LTα1β2) as a novel mediator downstream of TL1A that, along with interleukin (IL)-22, induces a PFD-associated signature in rectal fibroblast and epithelial cells, respectively. This signature includes an increased abundance of fibroblasts, an induction of matrix-degrading enzymes, transcriptomic rewiring of the lamina propria S1 fibroblasts and an anti-bacterial and immune responses in epithelial cells.
Notably, the induction of LTα1β2 and IL-22 occurs independently of tumour necrosis factor (TNF) signalling, revealing a new TL1A-LTα1β2/IL-22 axis that remains active under anti-TNF therapy.
ConclusionsOur findings revealed unique cellular alterations in the rectum of patients with CD+PFD, highlighting the previously unrecognised involvement of TL1A in mediating this signature and supporting the need for exploring the role of TL1A inhibition as a therapeutic approach for PFD.