T cells subset analysis
Last compiled on 17 November, 2022
We considered five major cell subsets: (1) myeloid, (2) T, (3) B and plasma, (4) stromal, and (5) epithelial cells. We classified cells into these subsets according to their gene expression. We consider in this file the cells classified as T cells. We reanalyze and QC the data, and look for the refined clusters.
Load libraries
library(Seurat)
library(plyr)
library(dplyr)
library(ggplot2)
library(DropletUtils)
library(celda)
library(SingleCellExperiment)
library(scater)
library(scran)
library(scDblFinder)
library(viridis)
library(MASS)
library(patchwork)
library(readr)
library(clustree)Load extra sources
get_density <- function(x, y, ...) { # function from https://slowkow.com/notes/ggplot2-color-by-density/
dens <- MASS::kde2d(x, y, ...)
ix <- findInterval(x, dens$x)
iy <- findInterval(y, dens$y)
ii <- cbind(ix, iy)
return(dens$z[ii])
}
fancy_scientific <- function(l) { # function from https://stackoverflow.com/a/24241954
# turn in to character string in scientific notation
l <- format(l, scientific = TRUE)
# quote the part before the exponent to keep all the digits
l <- gsub("^(.*)e", "'\\1'e", l)
# turn the 'e+' into plotmath format
l <- gsub("e", "%*%10^", l)
# return this as an expression
parse(text=l)
}
setwd("~/000_GitHub/ibd-bcn_single_cell")
source('source/functions_scrnaseq.R')
source('source/colors.R')Load the data
setwd('~/000_GitHub/ibd-bcn_single_cell/Analysis of our data/02_Samples_Together/')
tcells <- readRDS('SUBSETS/FROM_SAMPLES_TOGETHER/tcells.RDS')
cycling <- readRDS('SUBSETS/FROM_SAMPLES_TOGETHER/cycling.RDS')
tcells <- merge(tcells, cycling)Reanalysis
These are the clusters we selected as T cells and cycling cells in the “02_Data_to_subsets” file. Cycling cells were a compendium of T, B, plasma cells. We will remove the cells not belonging to this subset during the QC.
QC
MT-gene expression
Distribution of cells in scatter plot (nfeatures/percent.mt) to check where are the majority of our cells. We can see the dots colored by density using the function get_density shown in [Load extra functions and sources].
meta <- tcells@meta.data
meta$density <- get_density(meta$percent.mt, meta$nFeature_RNA, n = 100)
ggplot(meta) +
geom_point(aes(percent.mt, nFeature_RNA, color = density), size = 0.2) +
scale_color_viridis() + theme_classic() +
scale_y_log10()+
geom_vline(xintercept = 25, linetype = 2, color = 'gray')+
theme(text = element_text( size = 12),
axis.title = element_text( size = 12),
legend.text = element_blank())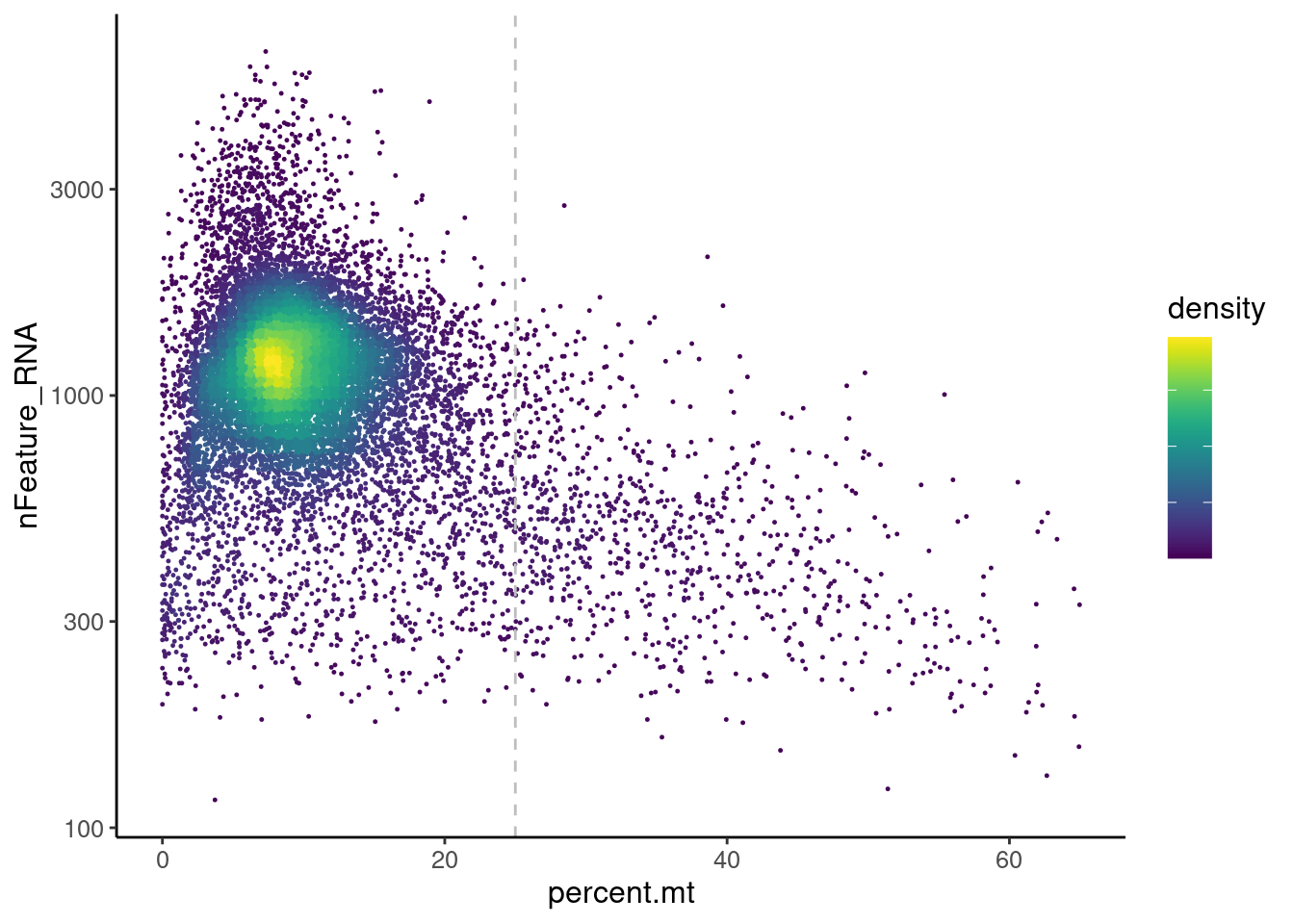 We analyzed the data using different cutoffs for the percent.mt (data
not shown) and finally decided that 25% was a reasonable choice for our
dataset.
We analyzed the data using different cutoffs for the percent.mt (data
not shown) and finally decided that 25% was a reasonable choice for our
dataset.
tcells <- tcells[,tcells$percent.mt < 25]Non-T cell genes
Right now tcells has 28287 features across 12345 cells.
Nevertheless, many cells express genes that we know should not be
expressed by T cells. We will remove those cells.
genes <- c('DERL3', 'MS4A1', 'C1QA', 'EPCAM', 'CD79A')
for (gene in genes) {
jd <- FetchData(tcells, vars = c('nCount_RNA', 'nFeature_RNA', gene))
jd <- jd[order(jd[,ncol(jd)]),]
k <- ggplot(jd, mapping = aes_string(x = 'nFeature_RNA', y = 'nCount_RNA', color = gene))+
geom_point() + theme_classic() + scale_color_viridis(alpha = 0.8) + scale_y_log10() + scale_x_log10()
cat("#### ", gene, "\n"); print(k); cat("\n\n")
}DERL3
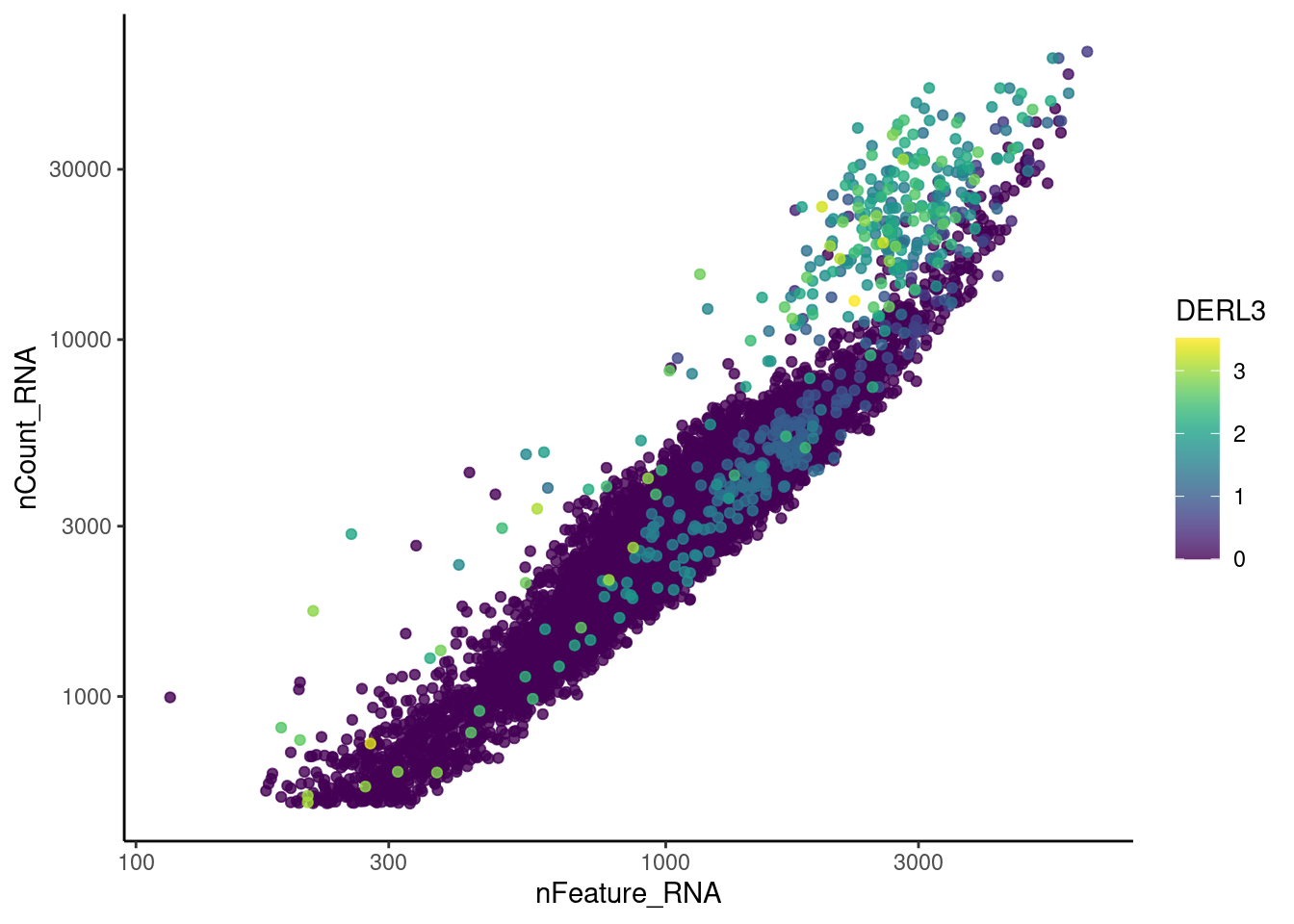
MS4A1
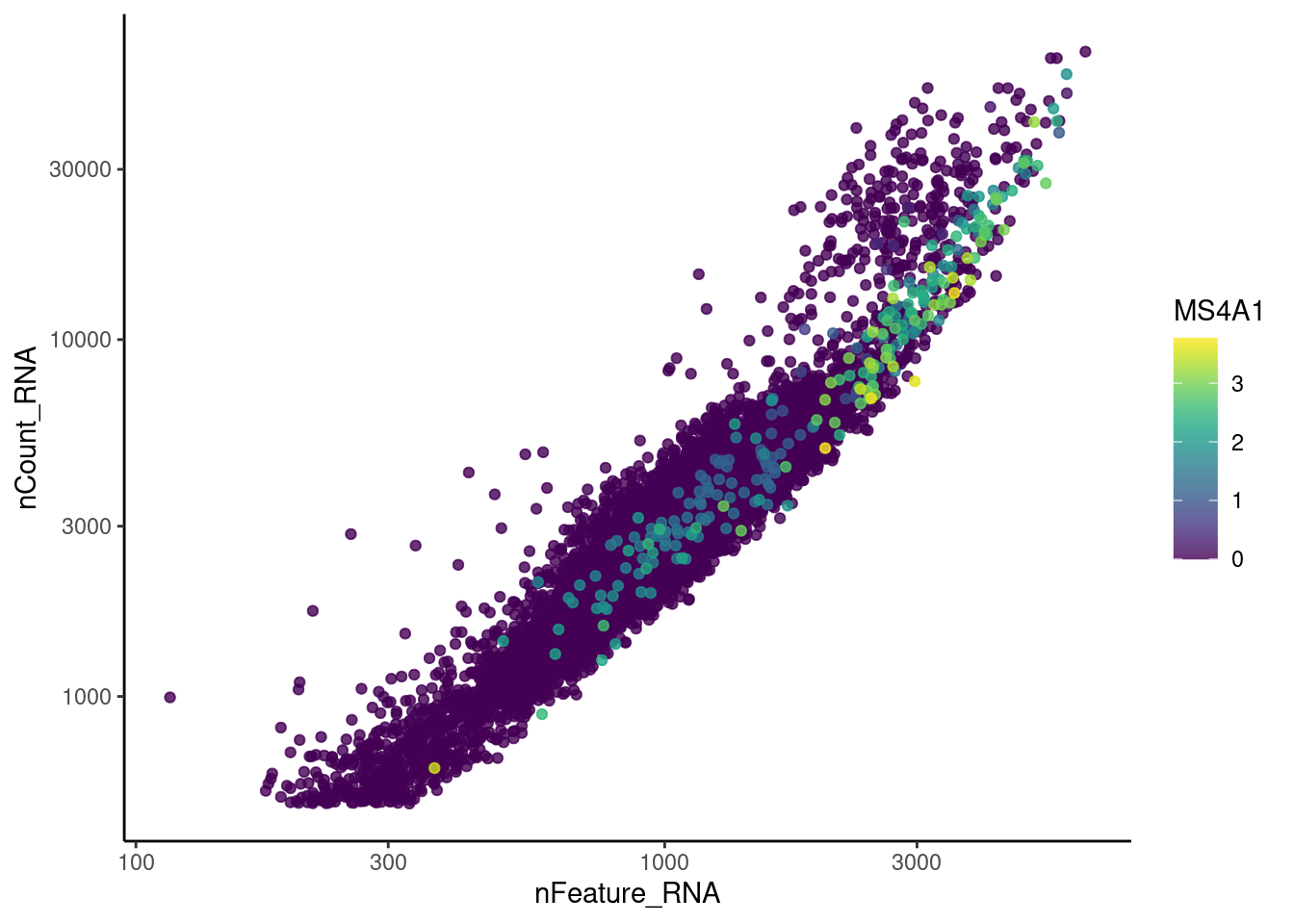
C1QA
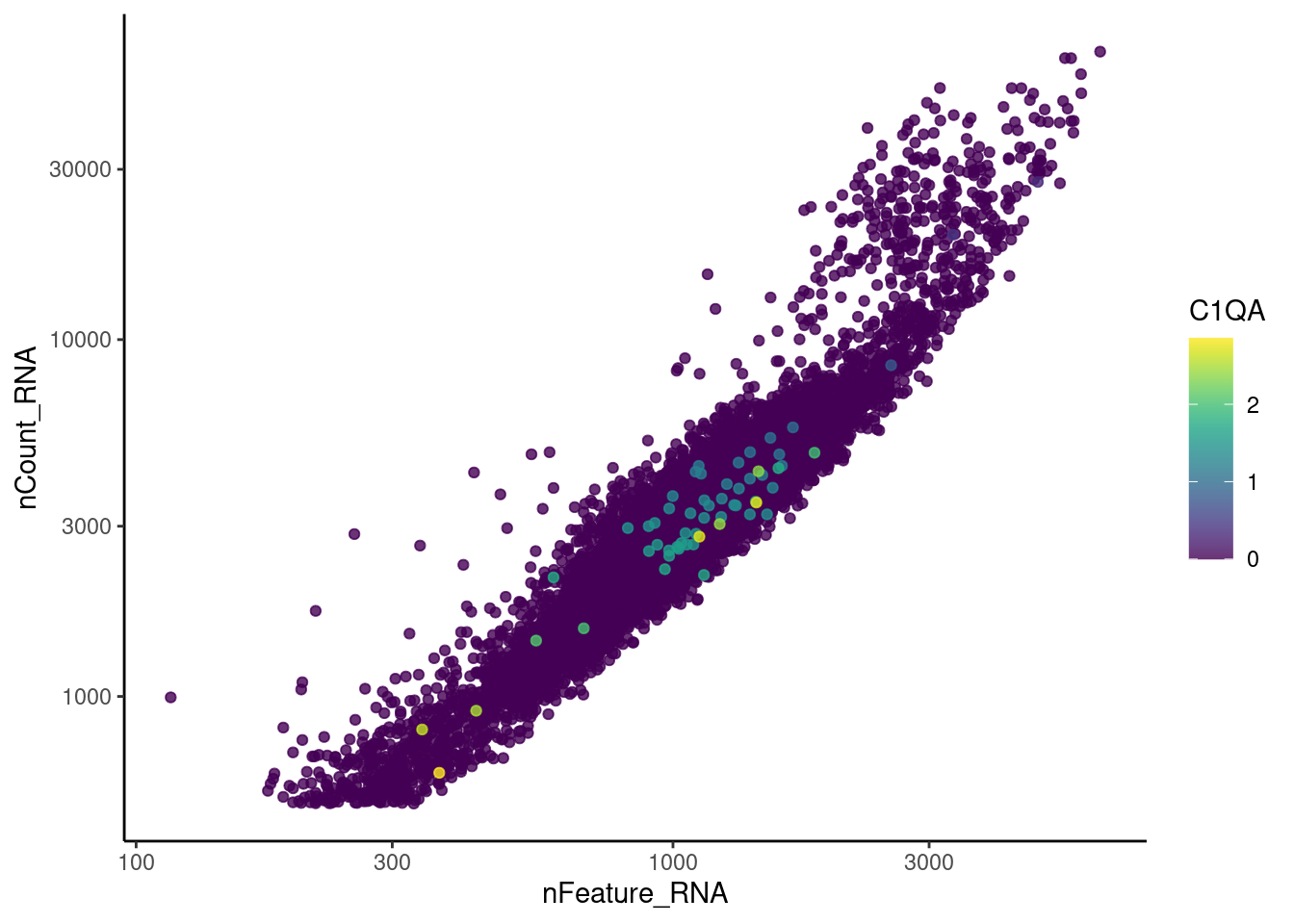
EPCAM
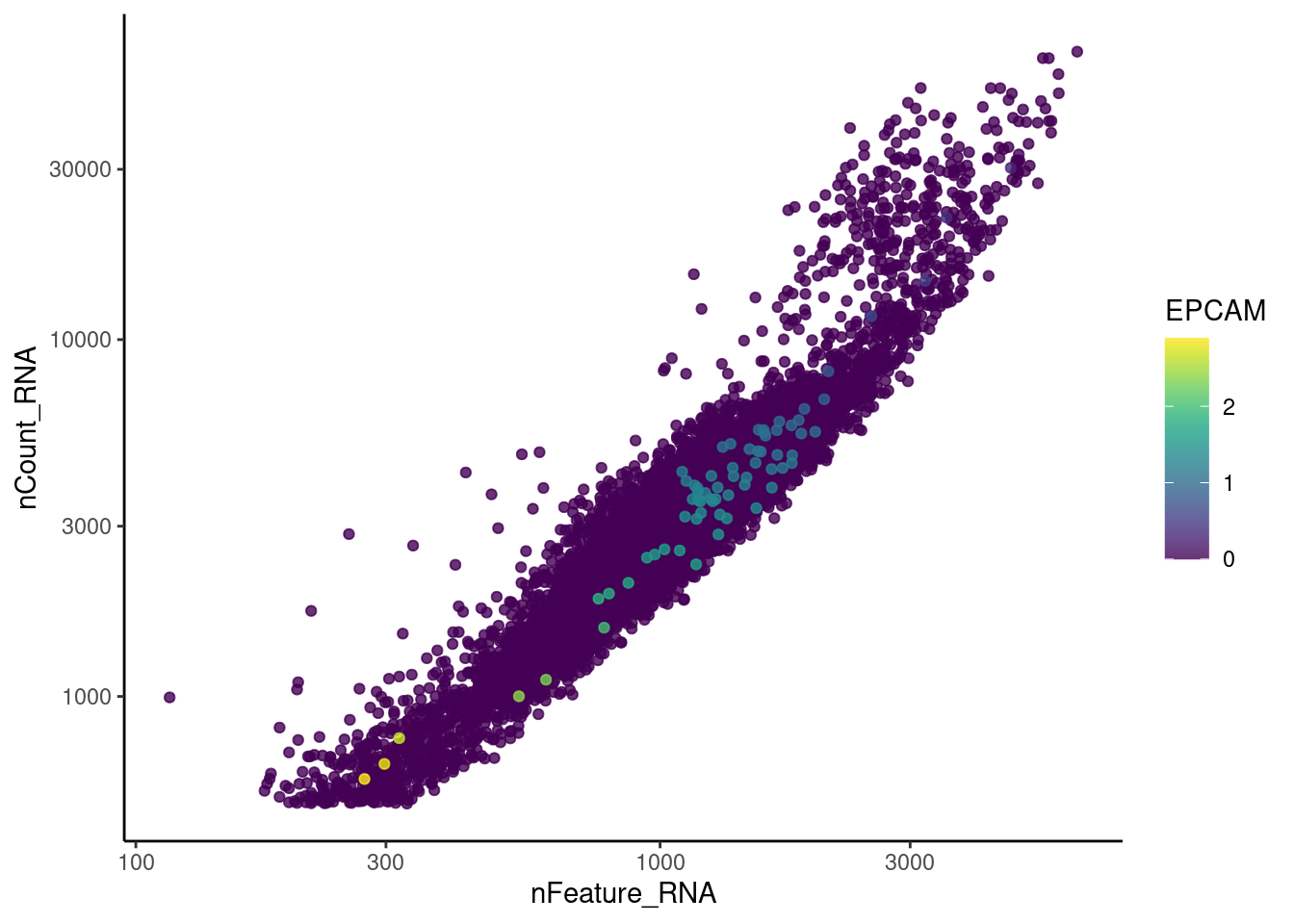
CD79A
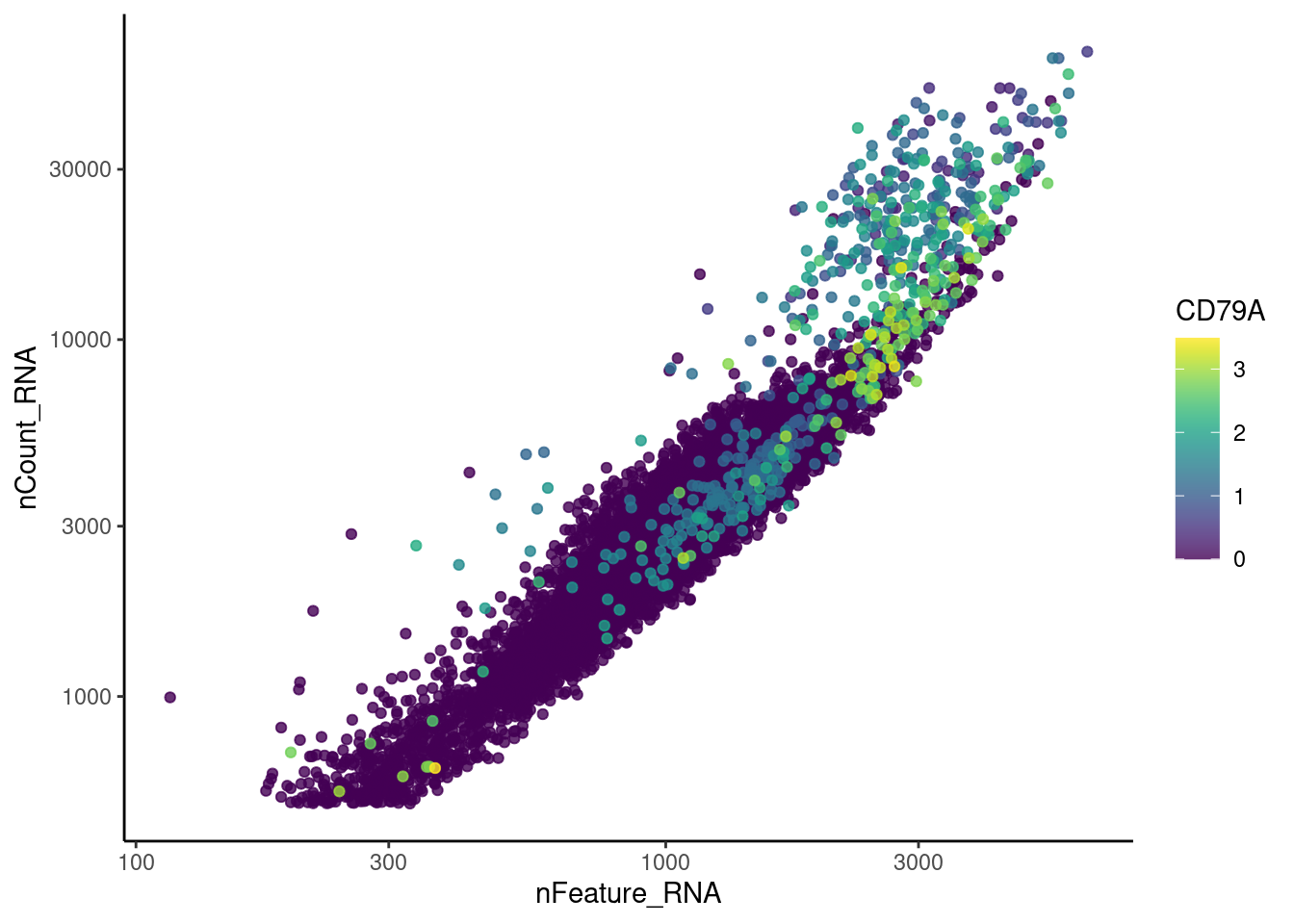
cat("#### code removal \n")code removal
counts <- tcells@assays$RNA@counts
p <- grep("^DERL3$|^MS4A1$|^C1QA$|^EPCAM$|^CD79A$",rownames(tcells))
pp <- which(Matrix::colSums(counts[p,])>0)
xx <-setdiff(colnames(tcells), names(pp))
tcells <- subset(tcells,cells = xx)
cat('\n\n')Ig genes signal
We have observed that due to normalization, there’s high Ig gene expression in cells that did not have high counts of Ig genes. As an example:
jc <- FetchData(tcells, vars = c('nCount_RNA', 'nFeature_RNA', 'IGHA1'), slot = 'counts')
jc <- jc[order(jc[,ncol(jc)]),]
jd <- FetchData(tcells, vars = c('nCount_RNA', 'nFeature_RNA', 'IGHA1'), slot = 'data')
jd <- jd[order(jd[,ncol(jd)]),]
kd <- ggplot(jd, mapping = aes_string(x = 'nFeature_RNA', y = 'nCount_RNA', color = 'IGHA1'))+
geom_point() + theme_classic() + scale_color_viridis(alpha = 0.8) +
scale_y_log10() + scale_x_log10() + labs(title = 'NormData')
kc <- ggplot(jc, mapping = aes_string(x = 'nFeature_RNA', y = 'nCount_RNA', color = 'IGHA1'))+
geom_point() + theme_classic() + scale_color_viridis(alpha = 0.8) +
scale_y_log10() + scale_x_log10() + labs(title = 'Counts')
wrap_plots(kd,kc, nrow = 1)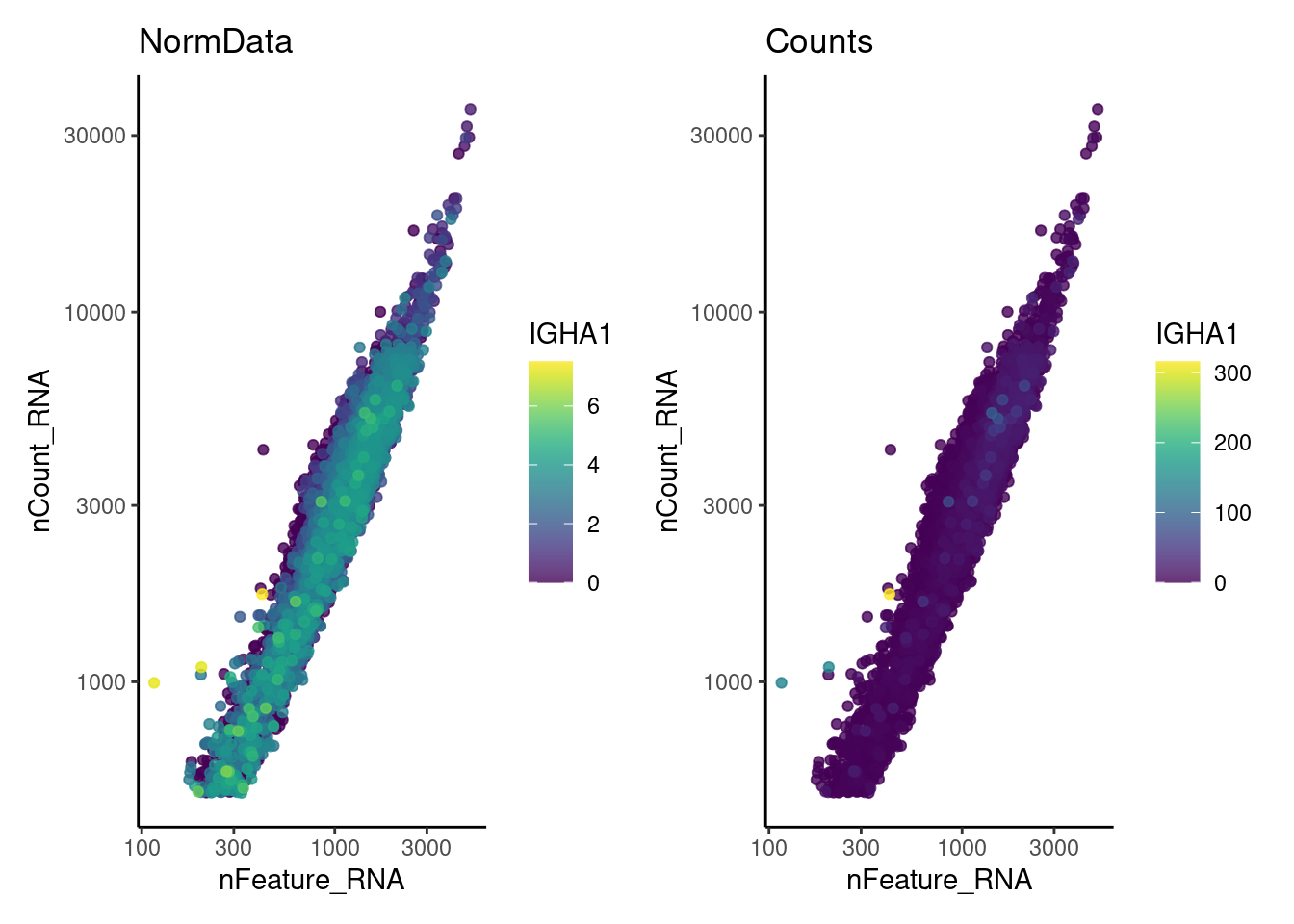
We remove the Ig genes from the dataset in all subsets except for the plasma and B cells subset.
gg <- rownames(tcells)[c(grep("^IGH",rownames(tcells)),
grep("^IGK", rownames(tcells)),
grep("^IGL", rownames(tcells)))]
genes <- setdiff(rownames(tcells),gg)
tcells <- subset(tcells,features = genes)Genes wo expression
We remove the genes without expression from the dataset.
counts <- tcells@assays$RNA@counts
pp <- which(Matrix::rowSums(counts)==0)
xx <-setdiff(rownames(tcells), names(pp))
tcells <- subset(tcells, features = xx)Dimension reduction
We tried different values of PC for the UMAP generation and Louvain clusterization. Finally, we considered 22 PCs for the analysis.
tcells <- seurat_to_pca(tcells)
a <- ElbowPlot(tcells, ndims = 100) +
geom_vline(xintercept = 25, colour="#BB0000", linetype = 2)+
labs(title = paste0('Elbowplot')) + theme_classic()
tcells<- FindNeighbors(tcells, dims = 1:22)
tcells<-RunUMAP(tcells, dims=1:22)
b <- DimPlot(tcells, group.by = 'sample_name') +
labs(title = '22 PCS') +
theme_classic() +
theme(legend.position = 'bottom')
(a+b) / guide_area() +
plot_layout(heights = c(0.7,0.3),guides = 'collect')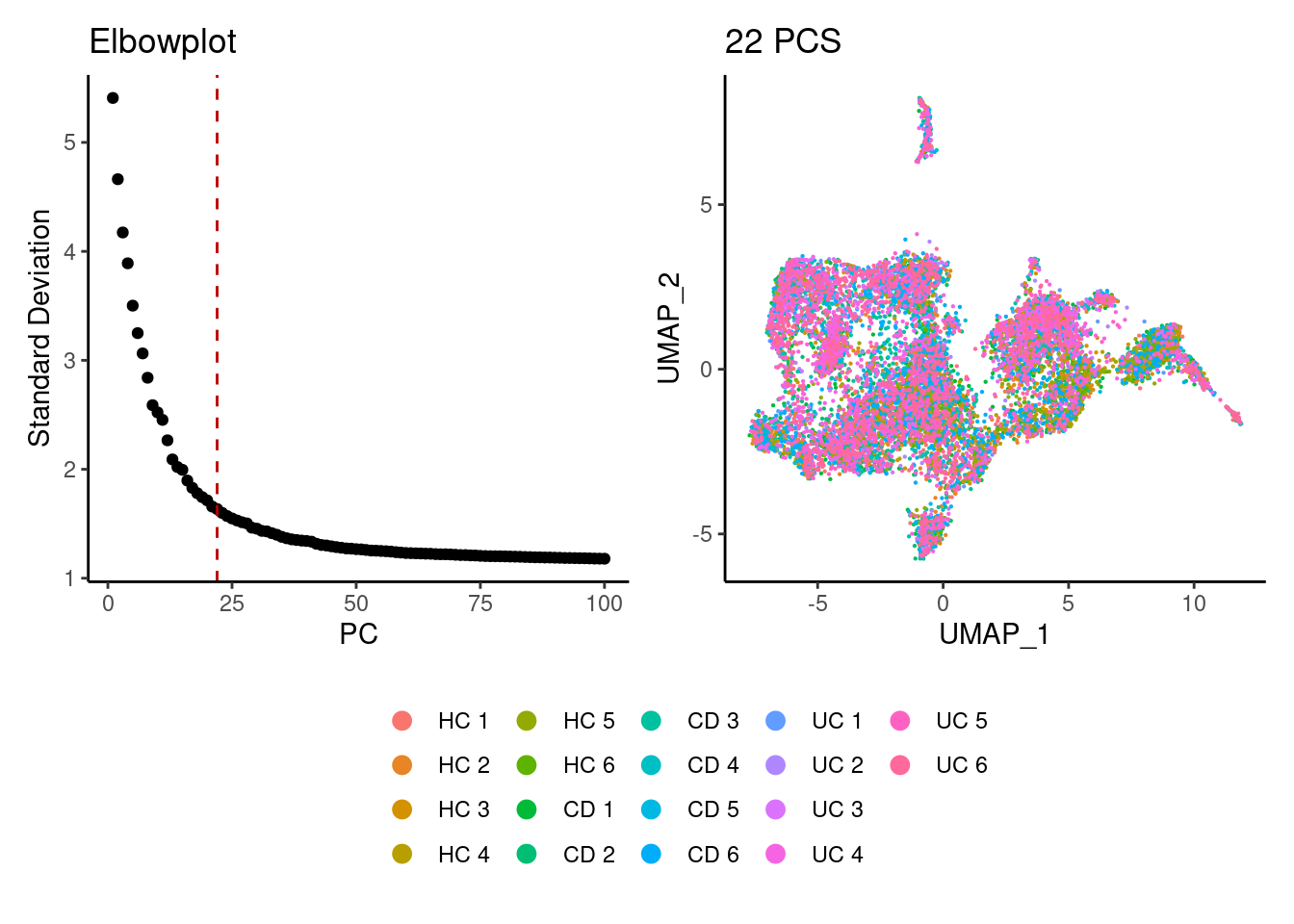
Harmony correction
tcells <- RunHarmony(tcells, group.by = 'sample', dims.use = 1:22)
a <- ElbowPlot(tcells, ndims = 100, reduction = 'harmony') +
geom_vline(xintercept = 40, linetype = 2)
tcells<- FindNeighbors(tcells, reduction = "harmony", dims = 1:40)
tcells<-RunUMAP(tcells, dims=1:40, reduction= "harmony")
DimPlot(tcells, group.by = 'sample_name') + theme_classic()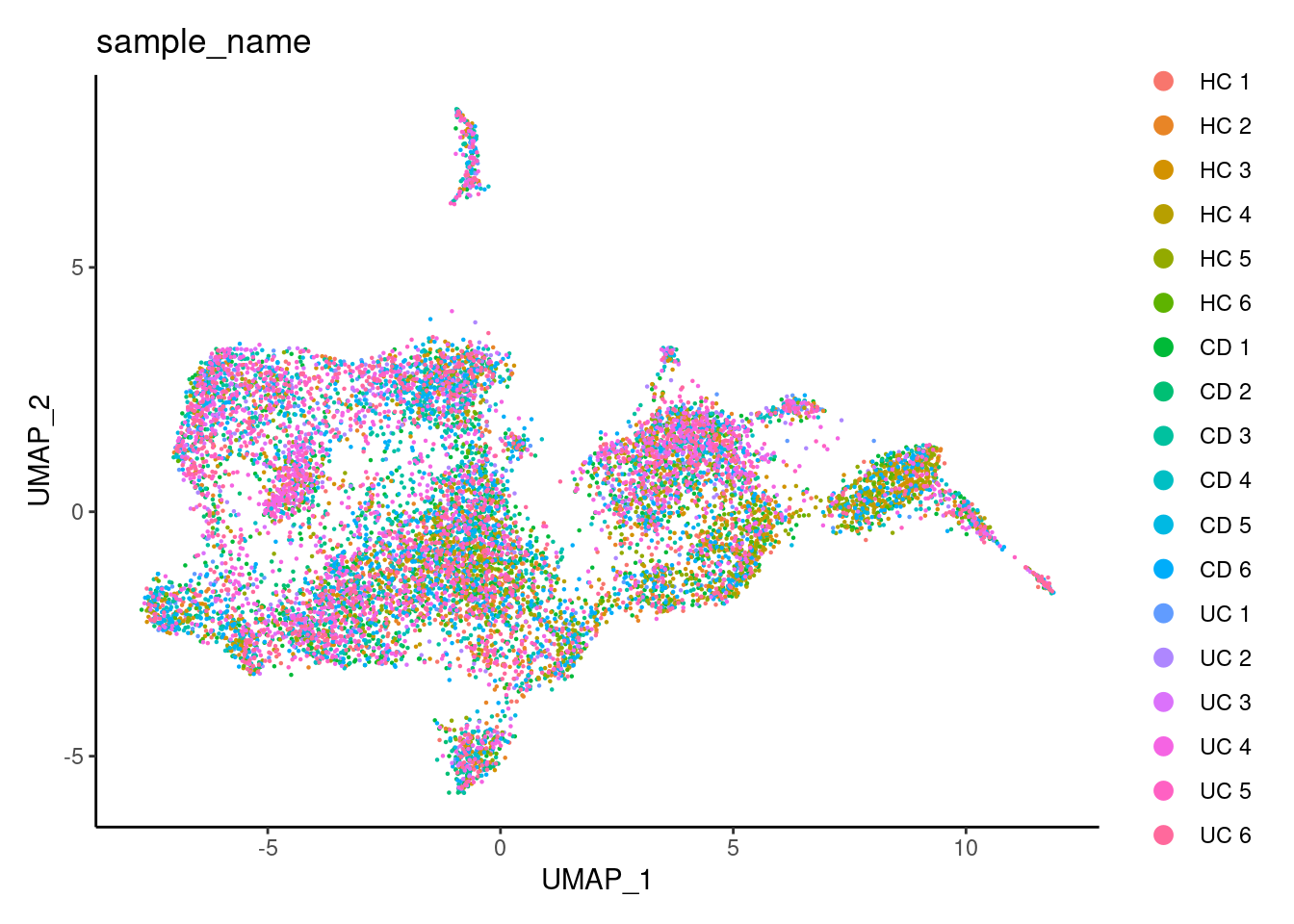
Louvain Clusterization
# dir.create('Markers')
# dir.create('Markers/Tcells')
path <- '~/000_GitHub/ibd-bcn_single_cell/Analysis of our data/02_Samples_Together/SUBSETS/Markers'
setwd(path)
tcells <- resolutions(tcells,
workingdir = path,
title = 'Tcells/Markers_tcells_')
path <- '~/000_GitHub/ibd-bcn_single_cell/Analysis of our data/02_Samples_Together/SUBSETS/ON_THEIR_OWN/'
setwd(path)
saveRDS(tcells, file = paste0(path,'tcells_filtered.RDS'))Dimplot Resolutions
path <- '~/000_GitHub/ibd-bcn_single_cell/Analysis of our data/02_Samples_Together/SUBSETS/ON_THEIR_OWN/'
tcells <- readRDS(file = paste0(path,'tcells_filtered.RDS'))
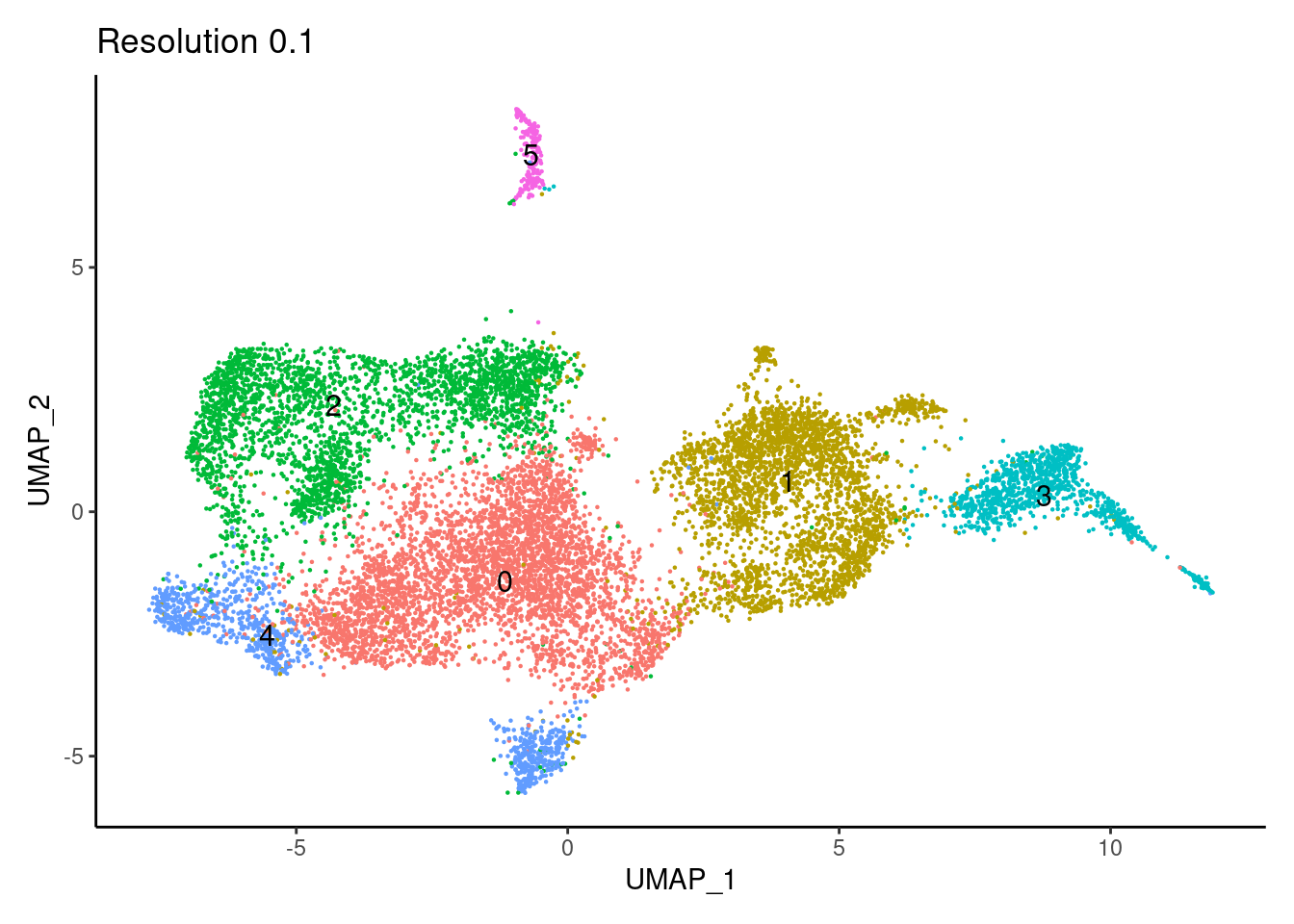
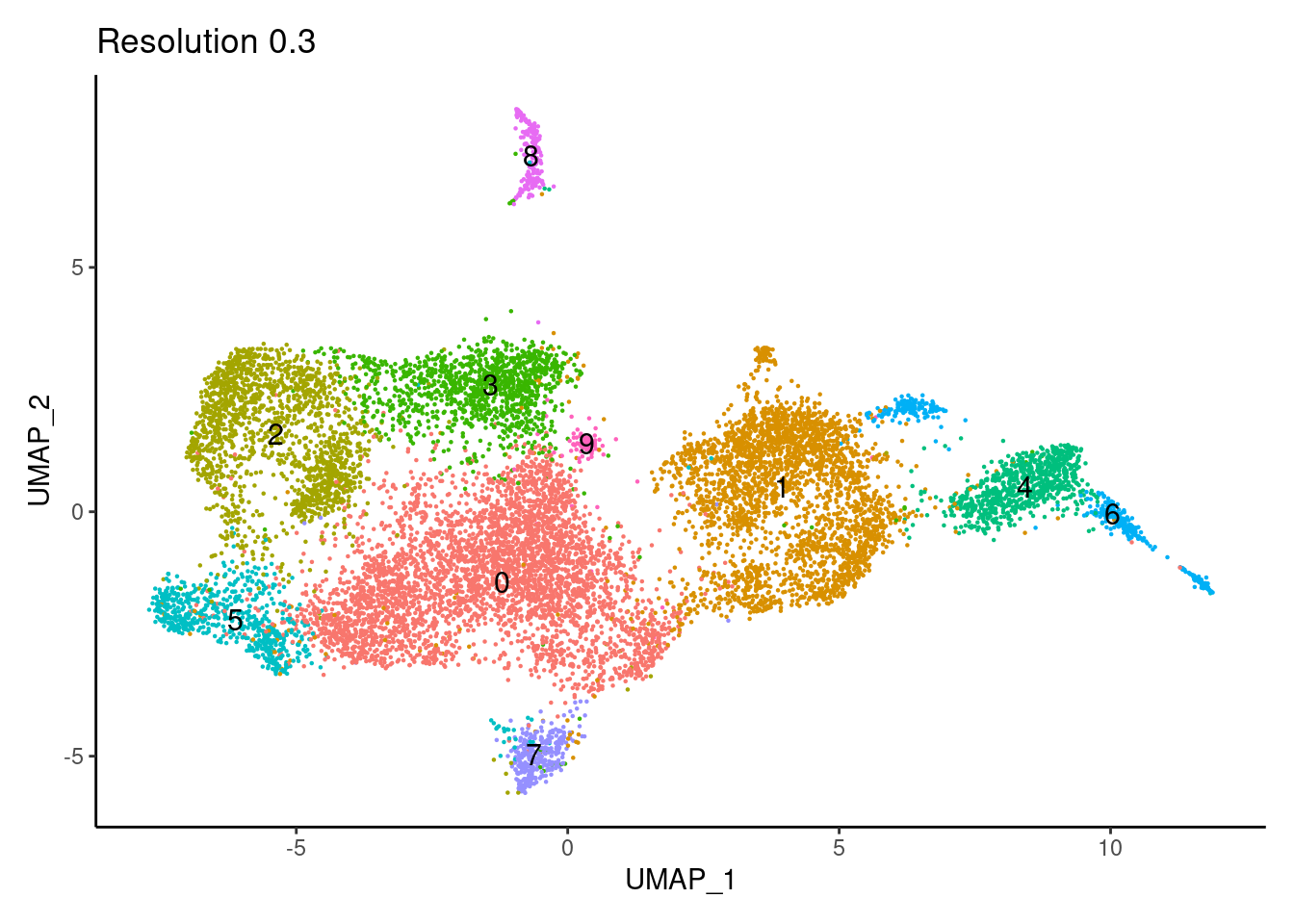
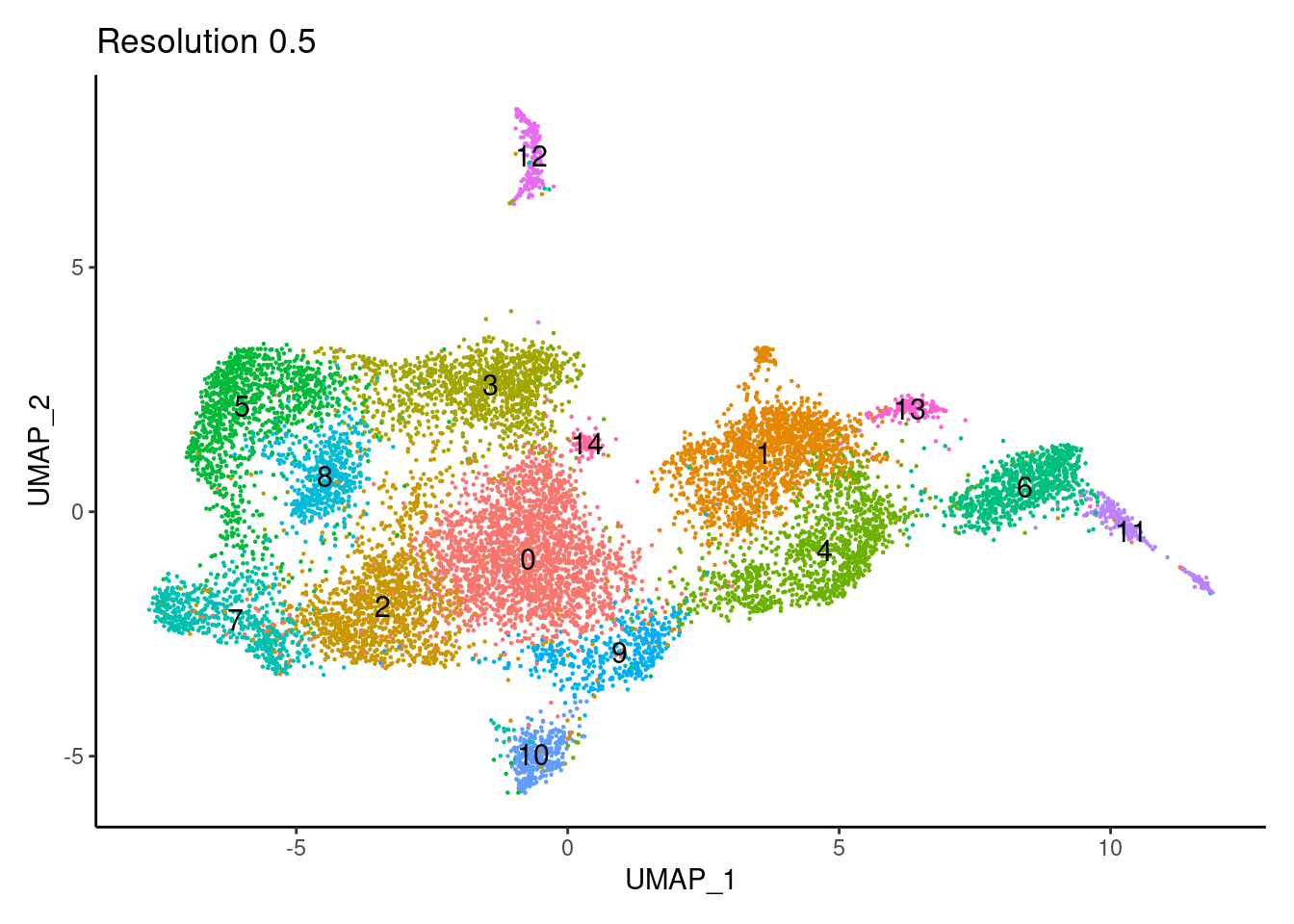
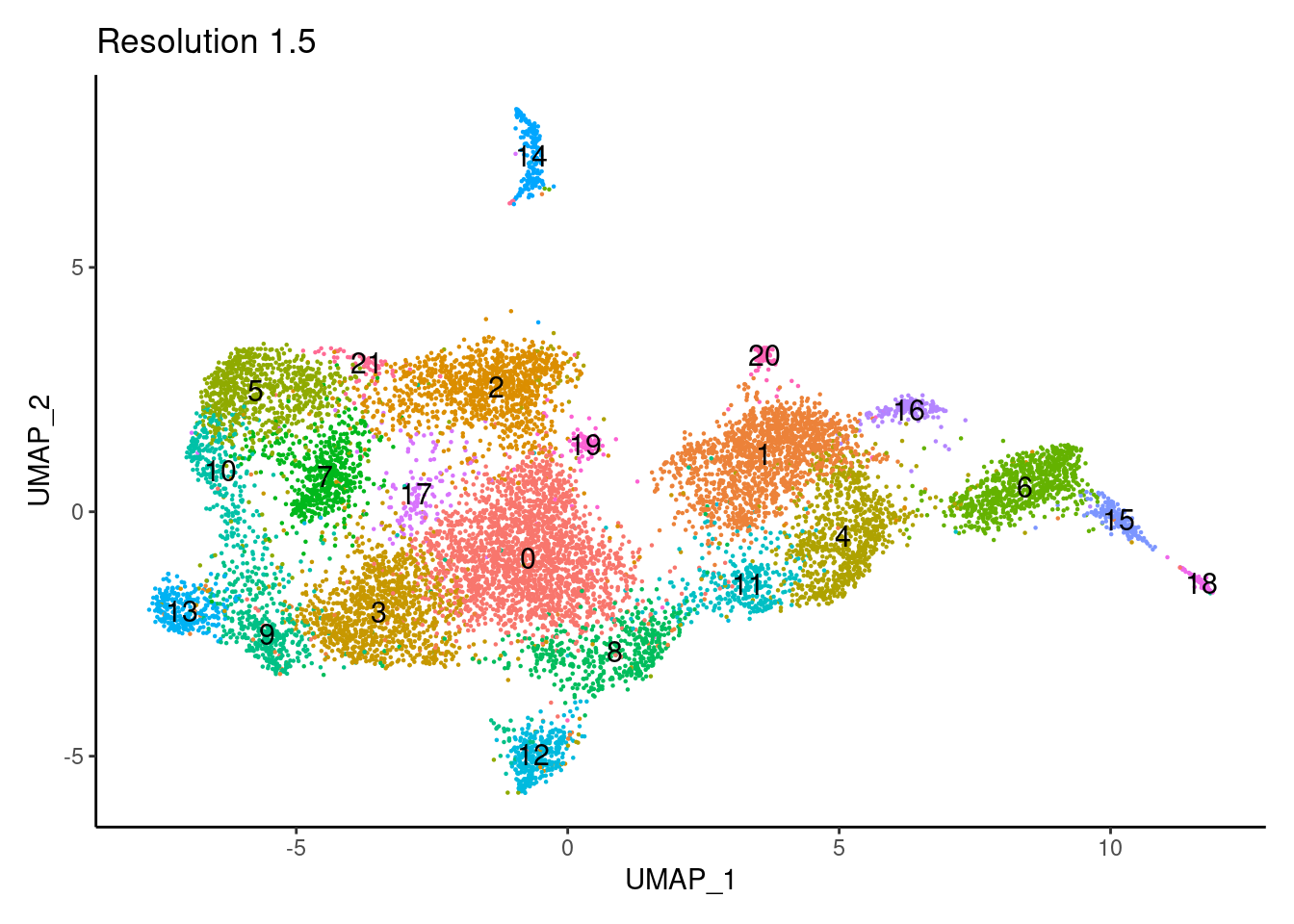
for(i in c('0.1','0.3','0.5','0.7','0.9','1.1','1.3','1.5')){
j <-DimPlot(tcells, group.by = paste0('RNA_snn_res.',i), label=T) +
theme_classic() +
theme(legend.position = 'none') +
labs(title = paste0('Resolution ',i))
cat("##### ", i, "\n"); print(j); cat("\n\n")
}0.1
0.3
0.5
0.7
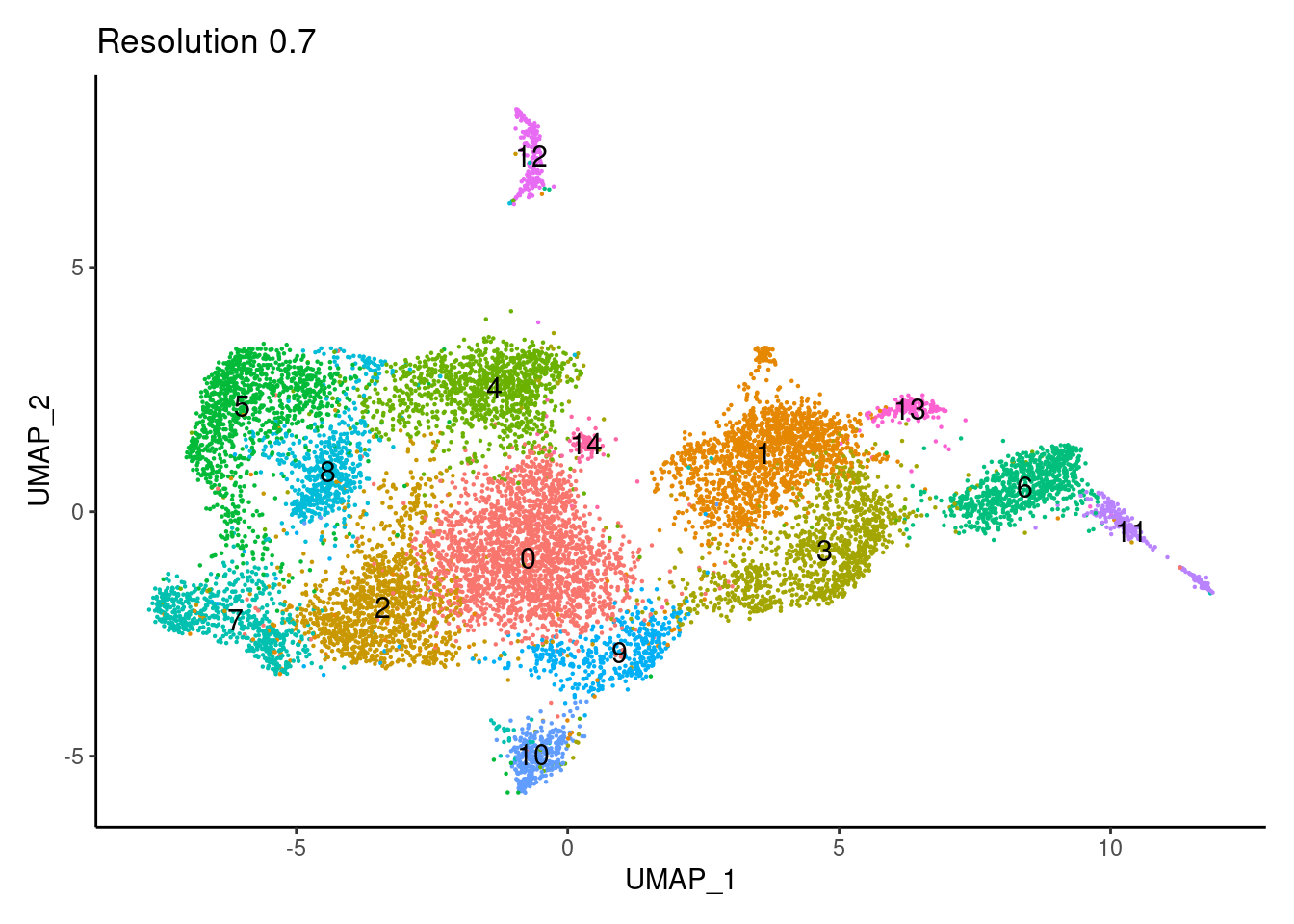
0.9
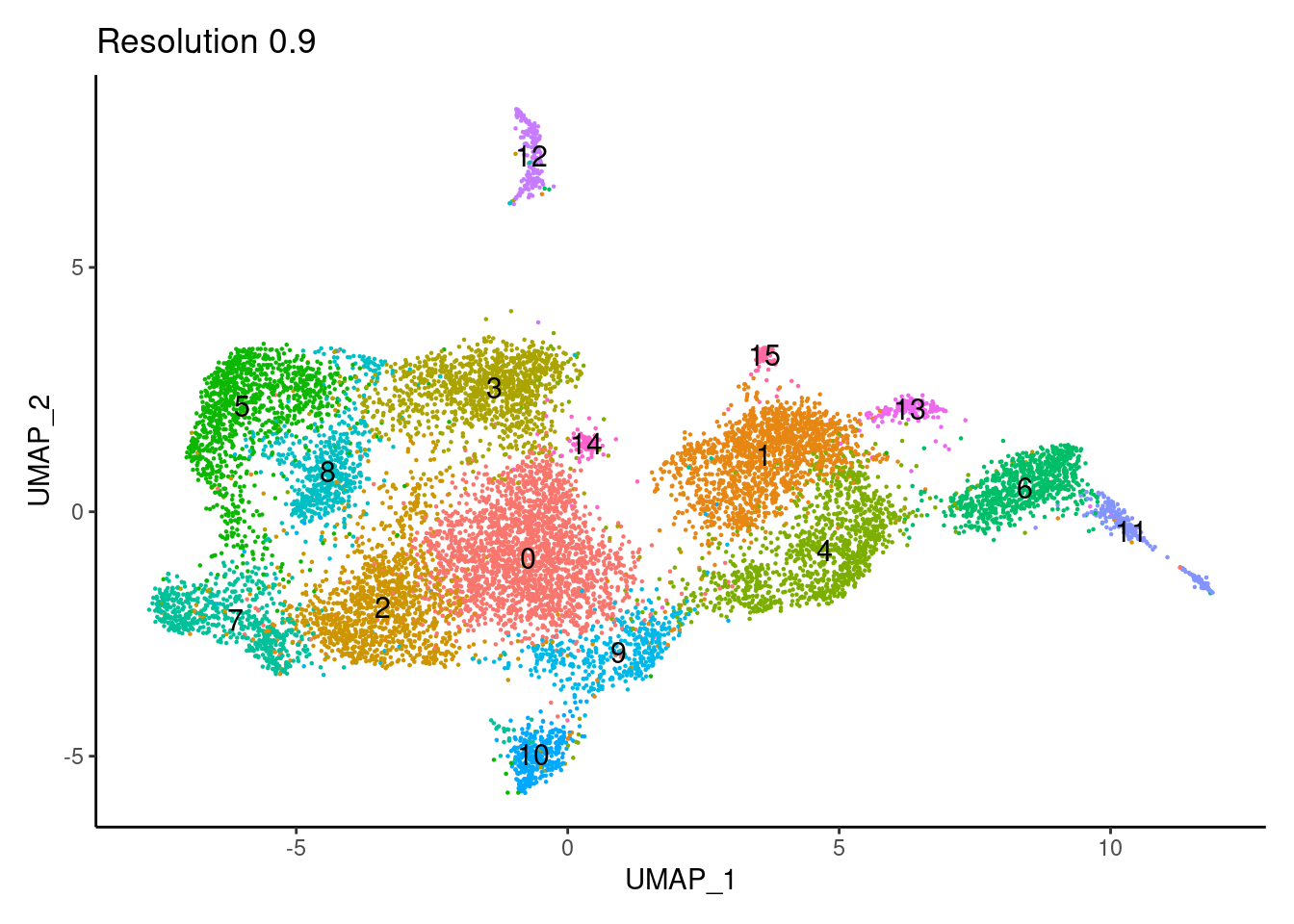
1.1
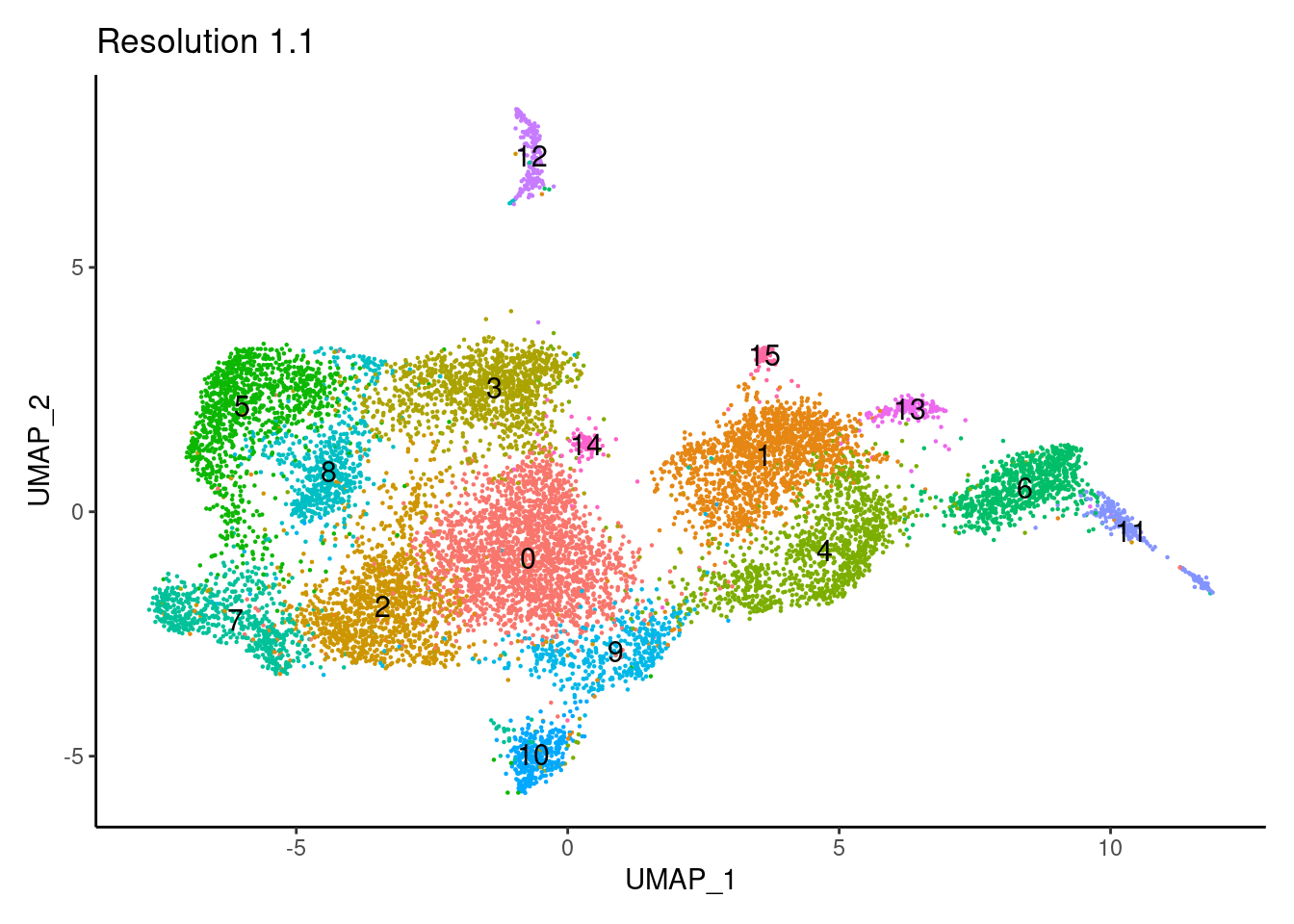
1.3
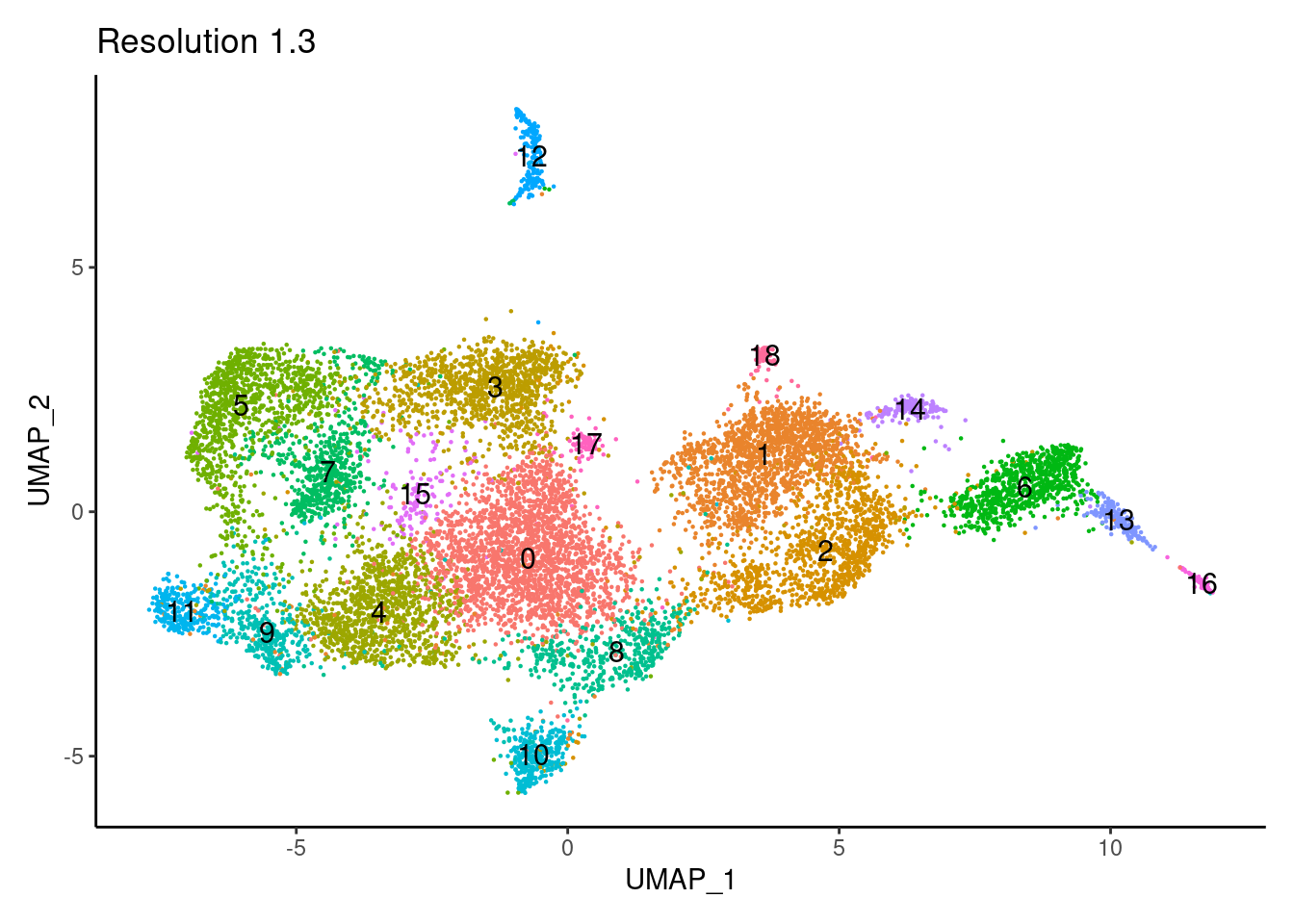
1.5
Clustree Resolutions
clustree(tcells, prefix = 'RNA_snn_res.') + guides(size = 'none', shape = 'none', edge_colour = FALSE, edge_alpha = FALSE) + theme(legend.position = 'right')## Warning: `guides(<scale> = FALSE)` is deprecated. Please use `guides(<scale> = "none")` instead.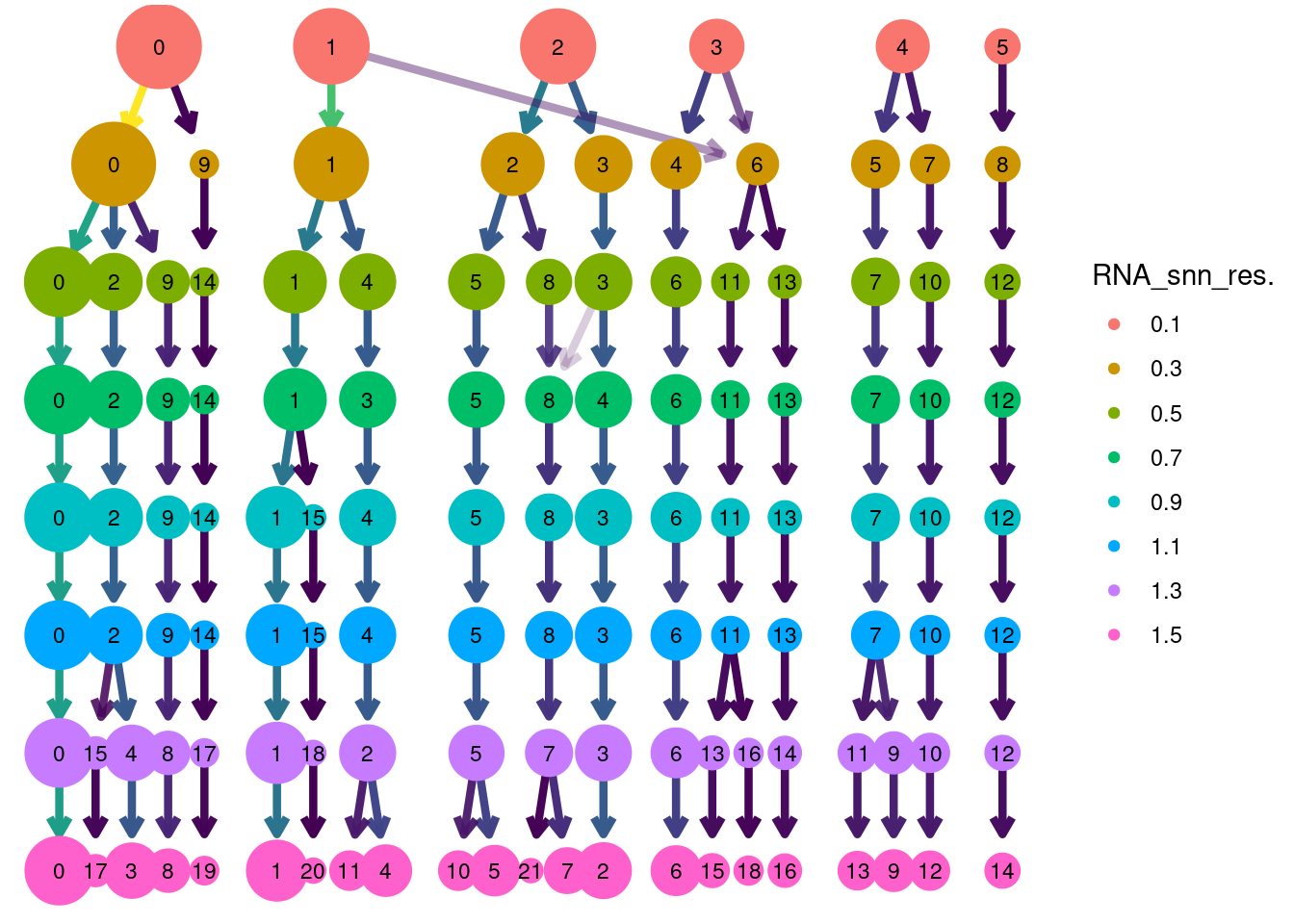
Top2 markers
setwd('~/000_GitHub/ibd-bcn_single_cell/Analysis of our data/02_Samples_Together/SUBSETS/')
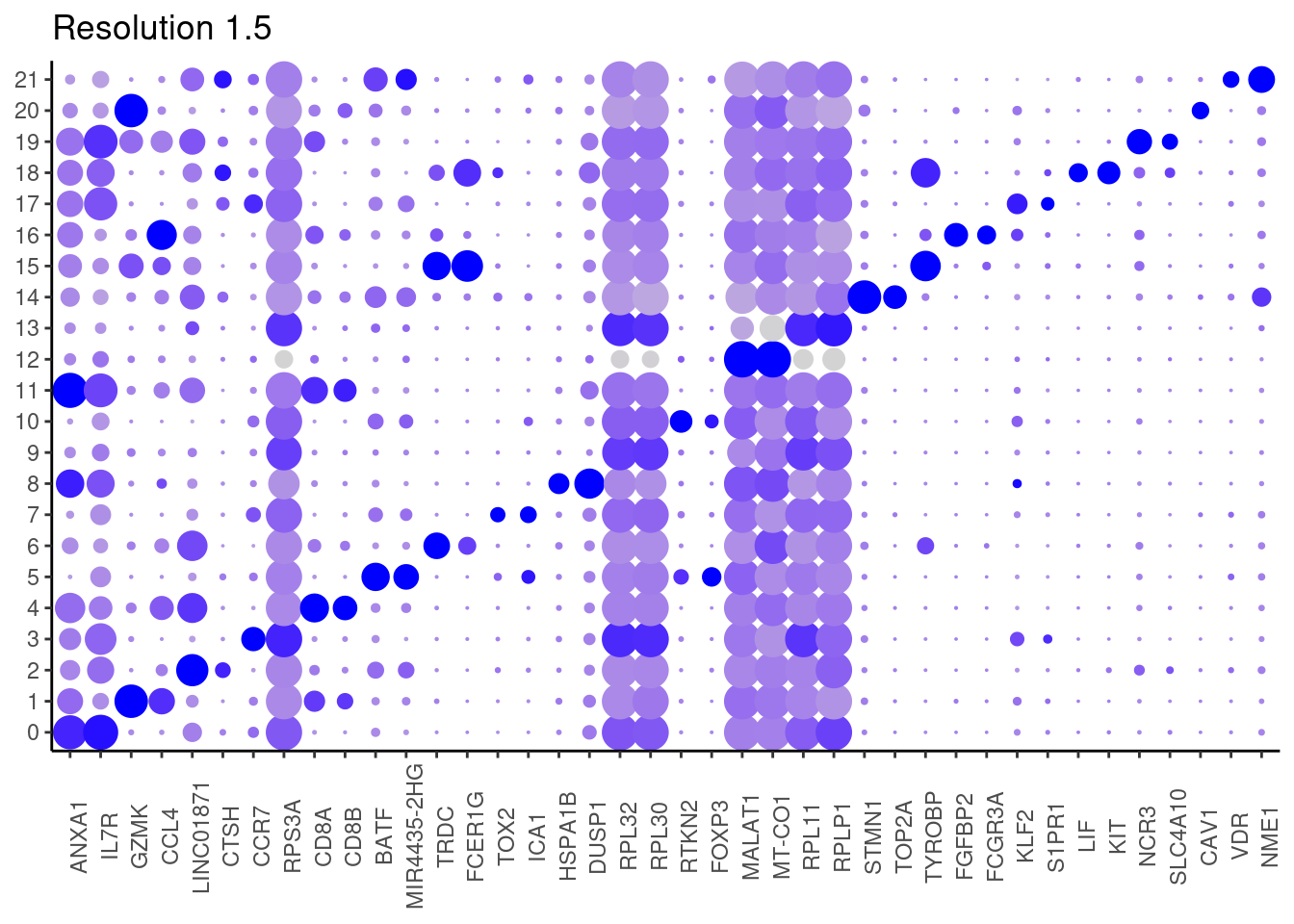
for(i in c('0.1','0.3','0.5','0.7','0.9','1.1','1.3','1.5')){
marker_genes <- read_delim(
paste0("Markers/Tcells/Markers_tcells__markers_resolution_",i,".csv"),
delim = ";", escape_double = FALSE, locale = locale(decimal_mark = ",",
grouping_mark = "."), trim_ws = TRUE)
top2 <- marker_genes %>%
dplyr::group_by(cluster)%>%
dplyr::slice(1:2)
top2_g <- unique(top2$gene)
j <- DotPlot(tcells, features = top2_g, group.by = paste0('RNA_snn_res.', i)) +
theme_classic() +
theme(axis.text.x = element_text(angle=90),
axis.title = element_blank()) +
NoLegend() +
labs(title = paste0('Resolution ',i))
cat("##### ", i, "\n"); print(j); cat("\n\n")
}## Rows: 1971 Columns: 7
## ── Column specification ──────────────────────────────────────────────────────────────────────────────────────────────
## Delimiter: ";"
## chr (1): gene
## dbl (6): p_val, avg_log2FC, pct.1, pct.2, p_val_adj, cluster
##
## ℹ Use `spec()` to retrieve the full column specification for this data.
## ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.0.1
## Rows: 2934 Columns: 7
## ── Column specification ──────────────────────────────────────────────────────────────────────────────────────────────
## Delimiter: ";"
## chr (1): gene
## dbl (6): p_val, avg_log2FC, pct.1, pct.2, p_val_adj, cluster
##
## ℹ Use `spec()` to retrieve the full column specification for this data.
## ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.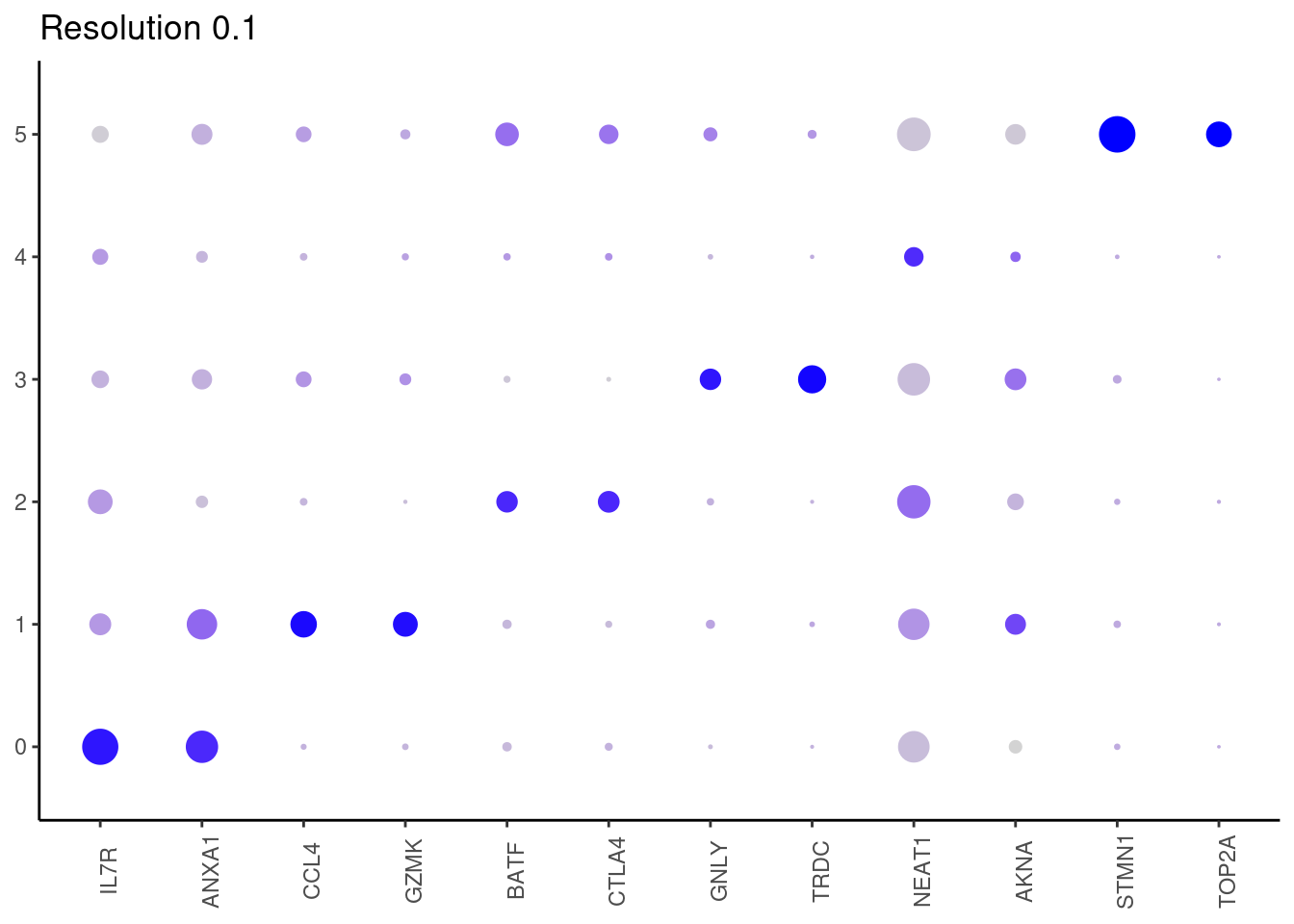 #####
0.3
#####
0.3
## Rows: 3862 Columns: 7
## ── Column specification ──────────────────────────────────────────────────────────────────────────────────────────────
## Delimiter: ";"
## chr (1): gene
## dbl (6): p_val, avg_log2FC, pct.1, pct.2, p_val_adj, cluster
##
## ℹ Use `spec()` to retrieve the full column specification for this data.
## ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.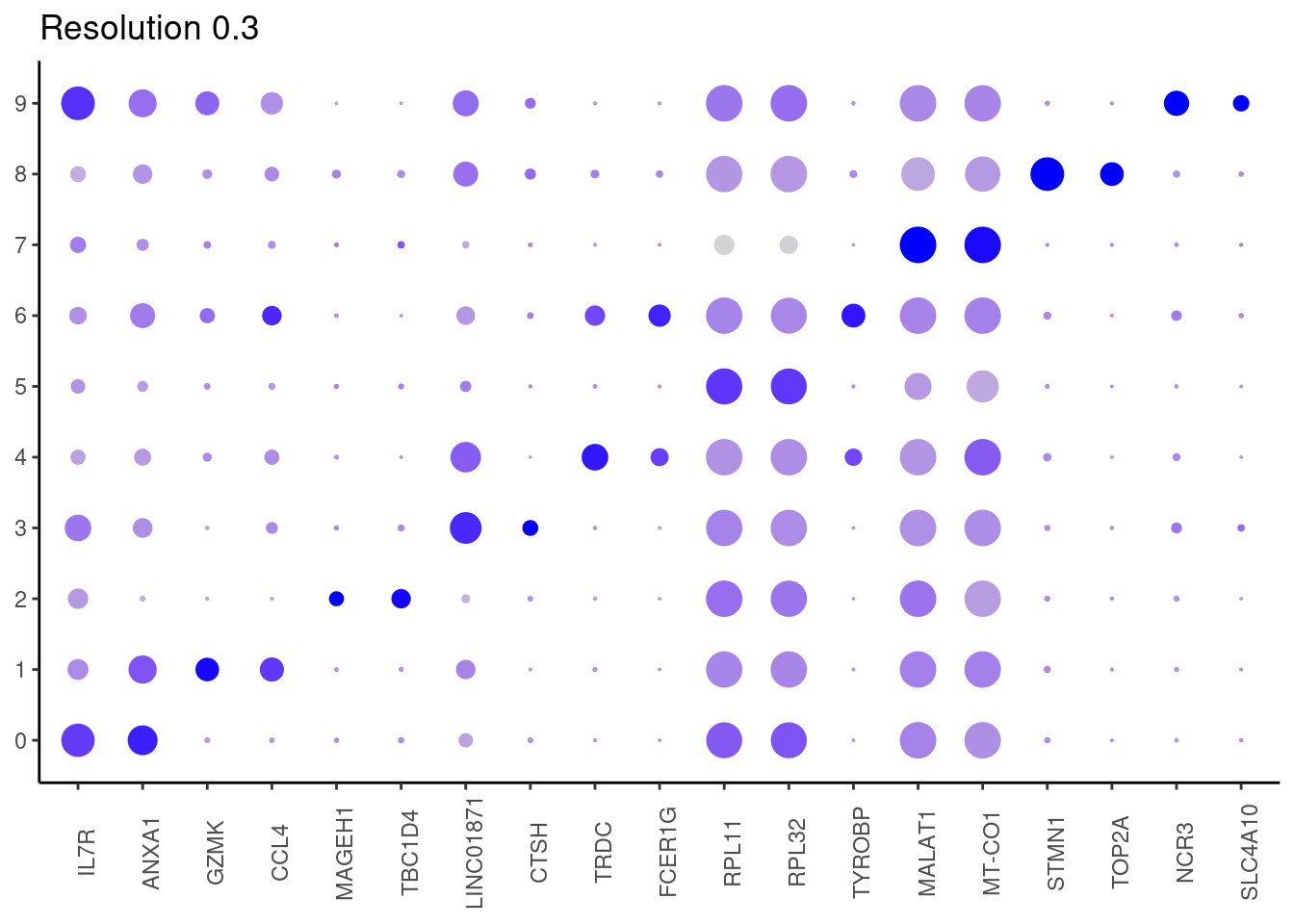 #####
0.5
#####
0.5
## Rows: 3855 Columns: 7
## ── Column specification ──────────────────────────────────────────────────────────────────────────────────────────────
## Delimiter: ";"
## chr (1): gene
## dbl (6): p_val, avg_log2FC, pct.1, pct.2, p_val_adj, cluster
##
## ℹ Use `spec()` to retrieve the full column specification for this data.
## ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.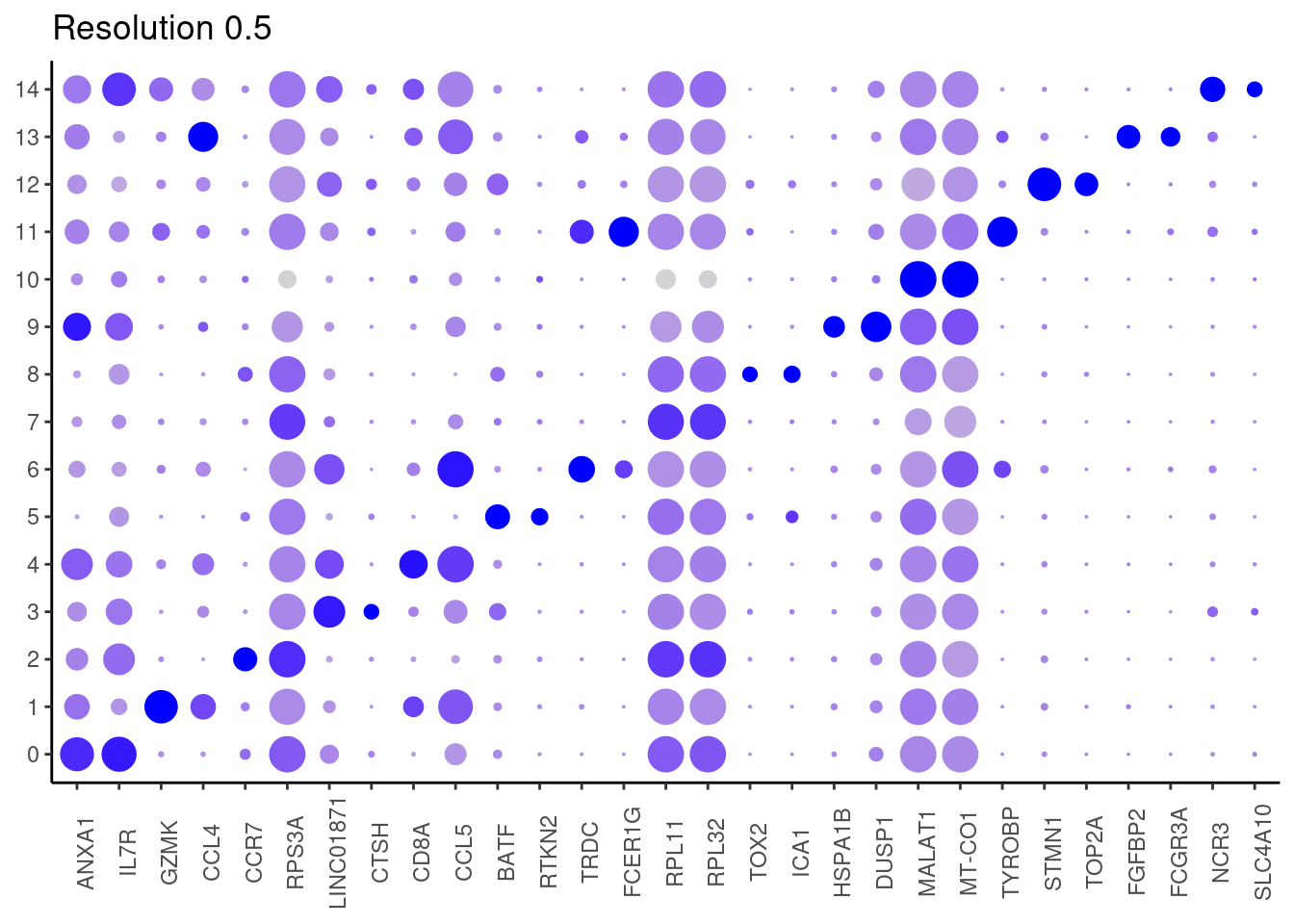 #####
0.7
#####
0.7
## Rows: 4230 Columns: 7
## ── Column specification ──────────────────────────────────────────────────────────────────────────────────────────────
## Delimiter: ";"
## chr (1): gene
## dbl (6): p_val, avg_log2FC, pct.1, pct.2, p_val_adj, cluster
##
## ℹ Use `spec()` to retrieve the full column specification for this data.
## ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.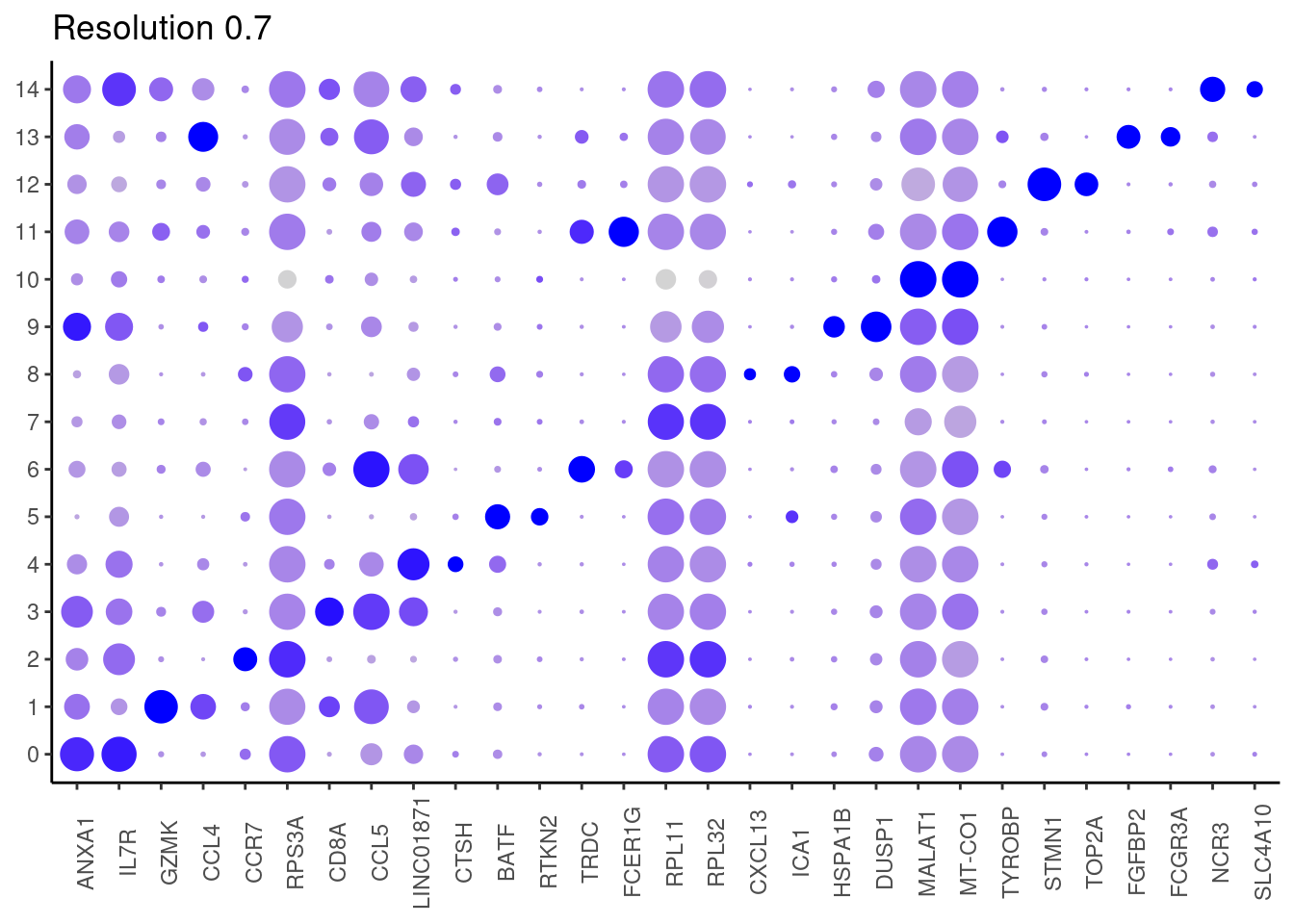 #####
0.9
#####
0.9
## Rows: 4217 Columns: 7
## ── Column specification ──────────────────────────────────────────────────────────────────────────────────────────────
## Delimiter: ";"
## chr (1): gene
## dbl (6): p_val, avg_log2FC, pct.1, pct.2, p_val_adj, cluster
##
## ℹ Use `spec()` to retrieve the full column specification for this data.
## ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.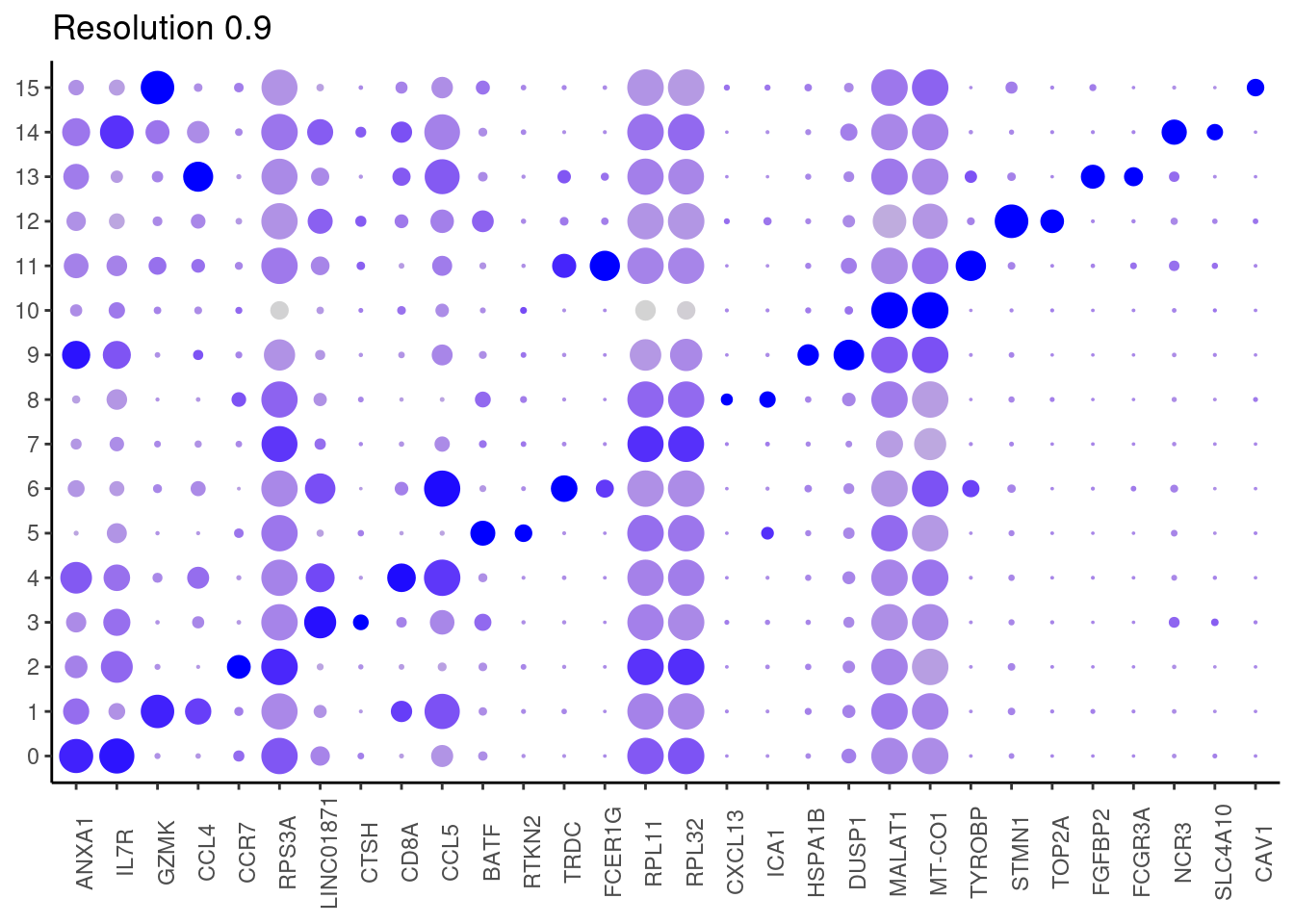 #####
1.1
#####
1.1
## Rows: 4937 Columns: 7
## ── Column specification ──────────────────────────────────────────────────────────────────────────────────────────────
## Delimiter: ";"
## chr (1): gene
## dbl (6): p_val, avg_log2FC, pct.1, pct.2, p_val_adj, cluster
##
## ℹ Use `spec()` to retrieve the full column specification for this data.
## ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.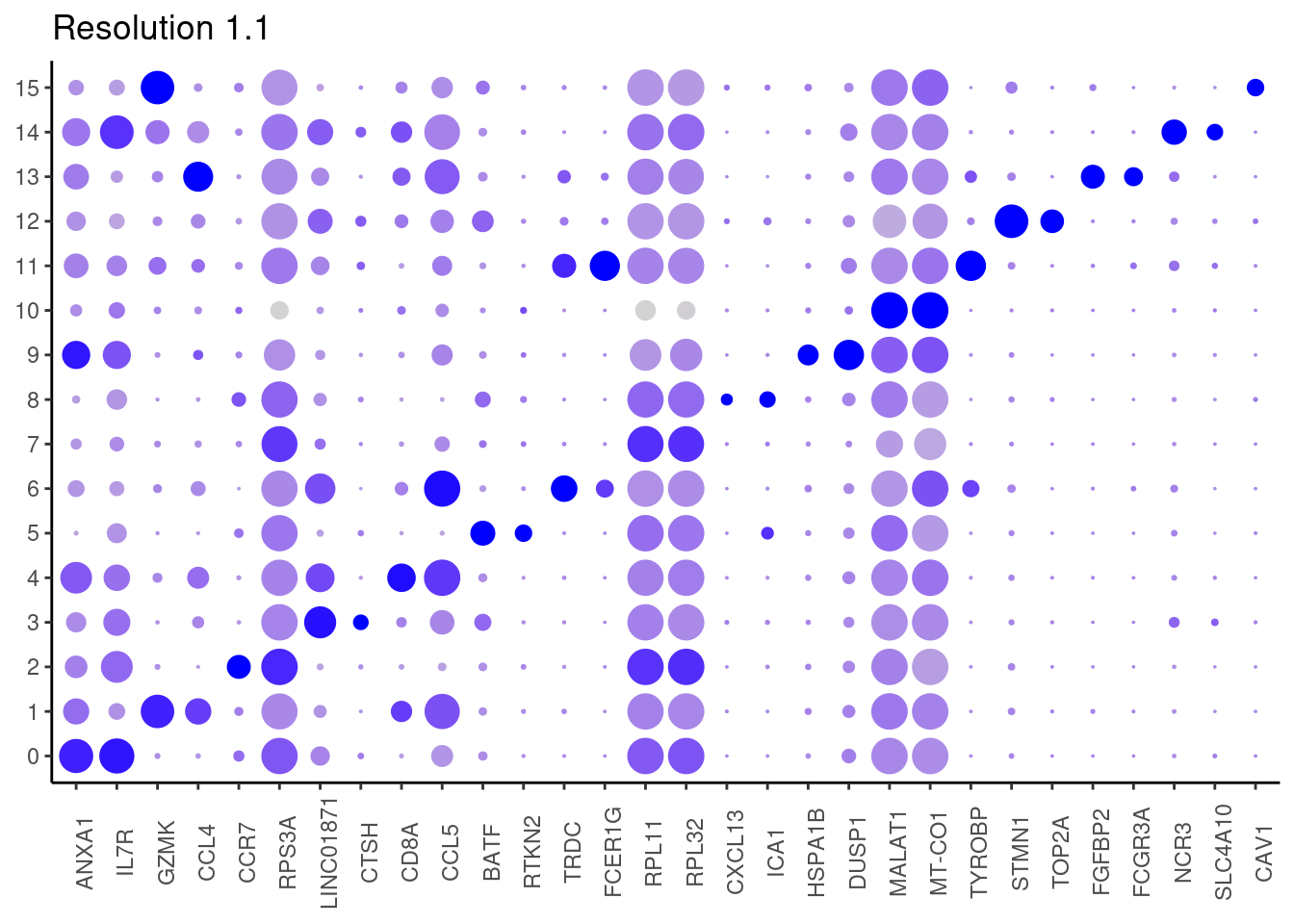 #####
1.3
#####
1.3
## Rows: 6289 Columns: 7
## ── Column specification ──────────────────────────────────────────────────────────────────────────────────────────────
## Delimiter: ";"
## chr (1): gene
## dbl (6): p_val, avg_log2FC, pct.1, pct.2, p_val_adj, cluster
##
## ℹ Use `spec()` to retrieve the full column specification for this data.
## ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.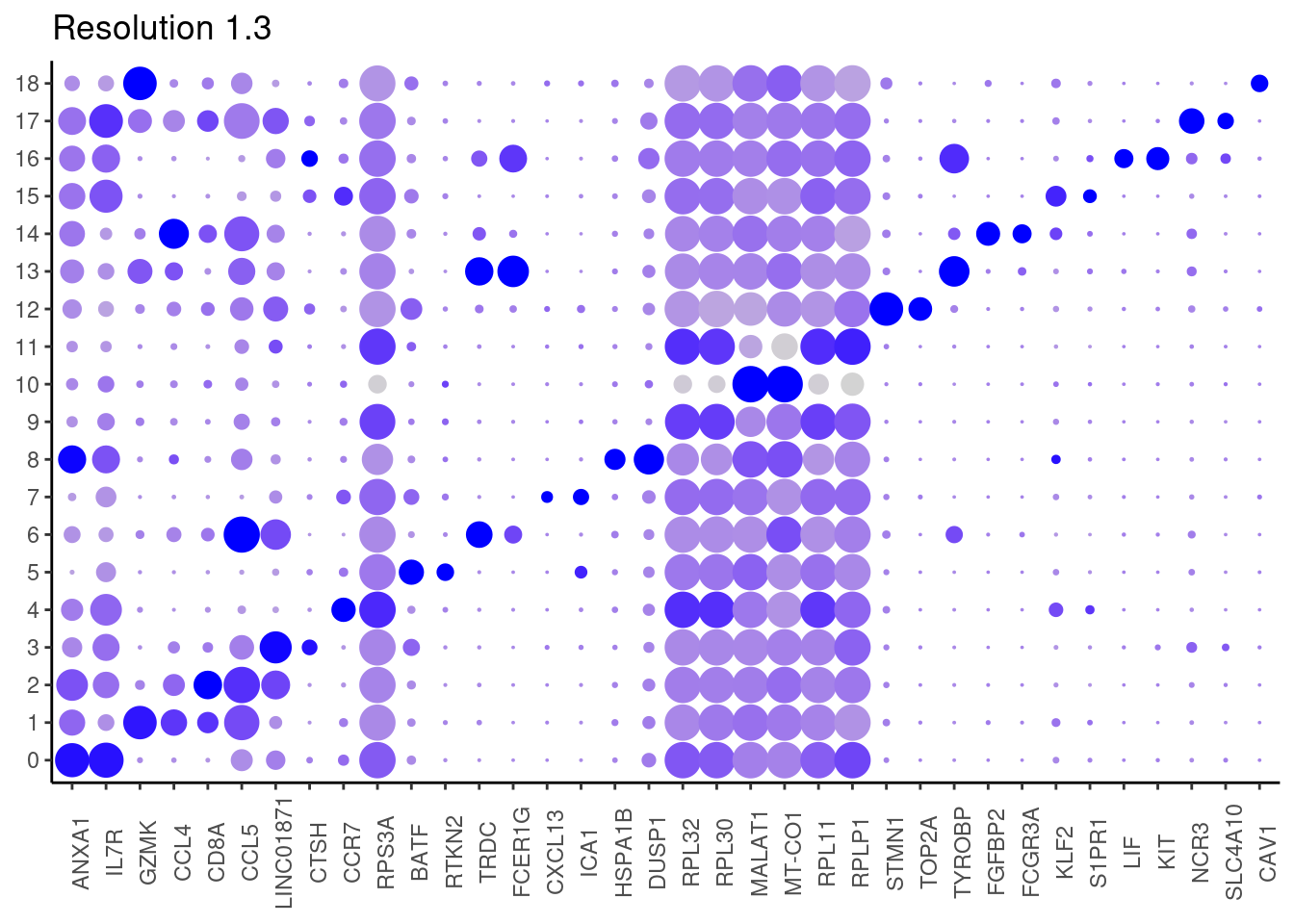 #####
1.5
#####
1.5
Annotation
tcells$annotation_refined <- plyr::mapvalues(x = tcells$RNA_snn_res.1.3,
from = 0:18,
to = c("CD4 ANXA1",
"CD8 CTL",
"CD8 CTL TRM",
"T cells CCL20",
"CD4 naïve",
"Tregs",
"gd IEL",
"ThF",
"DN TNF",
"Ribhi T cells 1",
"MT T cells",
"Ribhi T cells 2",
"Cycling T cells",
"NK",
"CD8 FGFBP2",
"S1PR1 T cells",
"ILC3",
"MAIT",
"DN EOMES"))
tcells$annotation_intermediate <- plyr::mapvalues(x = tcells$RNA_snn_res.1.3,
from = 0:18,
to = c("CD4",
"CD8",
"CD8",
"T cells CCL20",
"CD4",
"Tregs",
"gd IEL",
"CD4",
"DN",
"Ribhi T cells",
"MT T cells",
"Ribhi T cells",
"Cycling T cells",
"NK",
"CD8",
"CD4",
"ILC4",
"MAIT",
"DN"))
a <- DimPlot(tcells, group.by = 'annotation_intermediate', label = T, repel=T) + NoLegend()
b <- DimPlot(tcells, group.by = 'annotation_refined', label = T, repel=T, cols = cols_tcells) + NoLegend()
a+b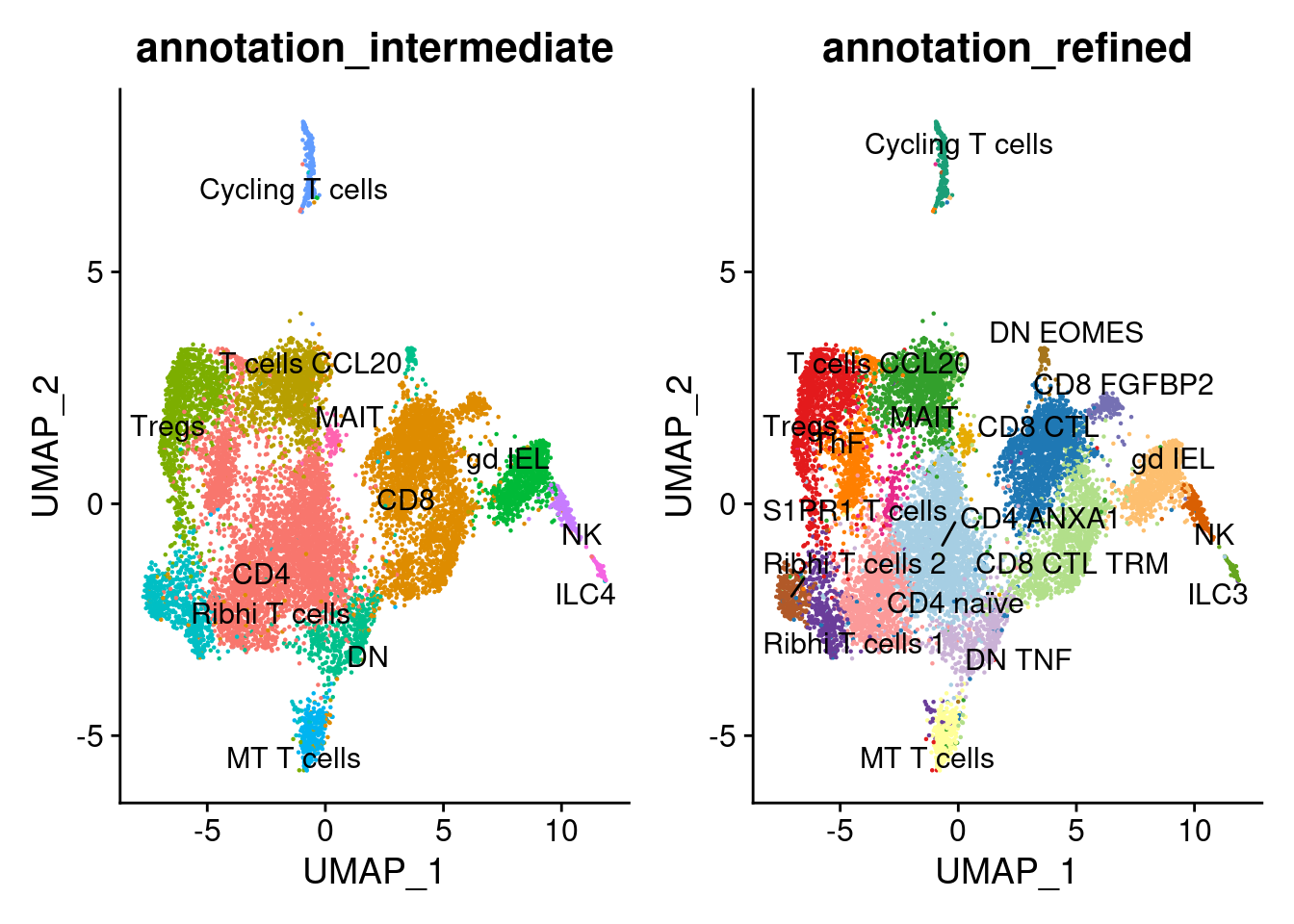
Save file
saveRDS(tcells, file = '~/000_GitHub/ibd-bcn_single_cell/Analysis of our data/02_Samples_Together/SUBSETS/ON_THEIR_OWN/tcells_annotated.RDS')
head(tcells@meta.data)## orig.ident nCount_RNA nFeature_RNA sample doublet Health newname Health_2 percent.mt
## SC_002_AAACCTGCAAGTCTGT-1 SC 2052 673 SC_002 singlet HC HC_SC_002 HC 4.180846
## SC_002_AAACCTGGTTATGCGT-1 SC 2724 897 SC_002 singlet HC HC_SC_002 HC 6.742396
## SC_002_AAACCTGTCGGCATCG-1 SC 3215 1108 SC_002 singlet HC HC_SC_002 HC 5.083695
## SC_002_AAACGGGGTCTAGCCG-1 SC 2927 958 SC_002 singlet HC HC_SC_002 HC 3.099455
## SC_002_AAACGGGGTGATAAAC-1 SC 2179 784 SC_002 singlet HC HC_SC_002 HC 5.205479
## SC_002_AAAGATGAGAGTCGGT-1 SC 3505 1038 SC_002 singlet HC HC_SC_002 HC 5.403868
## RNA_snn_res.0.1 seurat_clusters RNA_snn_res.0.3 RNA_snn_res.0.5 RNA_snn_res.0.7
## SC_002_AAACCTGCAAGTCTGT-1 0 3 0 2 2
## SC_002_AAACCTGGTTATGCGT-1 3 6 4 6 6
## SC_002_AAACCTGTCGGCATCG-1 1 4 1 4 3
## SC_002_AAACGGGGTCTAGCCG-1 0 0 0 0 0
## SC_002_AAACGGGGTGATAAAC-1 1 20 1 1 1
## SC_002_AAAGATGAGAGTCGGT-1 0 0 0 0 0
## RNA_snn_res.0.9 RNA_snn_res.1.1 RNA_snn_res.1.3 RNA_snn_res.1.5 sample_name
## SC_002_AAACCTGCAAGTCTGT-1 2 2 4 3 HC 1
## SC_002_AAACCTGGTTATGCGT-1 6 6 6 6 HC 1
## SC_002_AAACCTGTCGGCATCG-1 4 4 2 4 HC 1
## SC_002_AAACGGGGTCTAGCCG-1 0 0 0 0 HC 1
## SC_002_AAACGGGGTGATAAAC-1 15 15 18 20 HC 1
## SC_002_AAAGATGAGAGTCGGT-1 0 0 0 0 HC 1
## annotation_refined annotation_intermediate
## SC_002_AAACCTGCAAGTCTGT-1 CD4 naïve CD4
## SC_002_AAACCTGGTTATGCGT-1 gd IEL gd IEL
## SC_002_AAACCTGTCGGCATCG-1 CD8 CTL TRM CD8
## SC_002_AAACGGGGTCTAGCCG-1 CD4 ANXA1 CD4
## SC_002_AAACGGGGTGATAAAC-1 DN EOMES DN
## SC_002_AAAGATGAGAGTCGGT-1 CD4 ANXA1 CD4sessionInfo()
sessionInfo()## R version 4.1.2 (2021-11-01)
## Platform: x86_64-pc-linux-gnu (64-bit)
## Running under: Ubuntu 20.04.5 LTS
##
## Matrix products: default
## BLAS: /usr/lib/x86_64-linux-gnu/blas/libblas.so.3.9.0
## LAPACK: /opt/R/4.1.2/lib/R/lib/libRlapack.so
##
## locale:
## [1] LC_CTYPE=C.UTF-8 LC_NUMERIC=C LC_TIME=C.UTF-8 LC_COLLATE=C.UTF-8
## [5] LC_MONETARY=C.UTF-8 LC_MESSAGES=C LC_PAPER=C.UTF-8 LC_NAME=C
## [9] LC_ADDRESS=C LC_TELEPHONE=C LC_MEASUREMENT=C.UTF-8 LC_IDENTIFICATION=C
##
## attached base packages:
## [1] stats4 stats graphics grDevices utils datasets methods base
##
## other attached packages:
## [1] Matrix_1.4-0 nnet_7.3-17 matchSCore2_0.1.0 harmony_0.1.0
## [5] Rcpp_1.0.9 rmarkdown_2.18 pandoc_0.1.0 readxl_1.3.1
## [9] magick_2.7.3 data.table_1.14.2 BiocParallel_1.28.3 RColorBrewer_1.1-3
## [13] ggrepel_0.9.1 ggrastr_1.0.1 usethis_2.1.5 clustree_0.4.4
## [17] ggraph_2.0.5 readr_2.1.2 dplyr_1.0.10 cowplot_1.1.1
## [21] reshape_0.8.8 formulaic_0.0.8 patchwork_1.1.2 MASS_7.3-55
## [25] viridis_0.6.2 viridisLite_0.4.1 scDblFinder_1.8.0 scran_1.22.1
## [29] scater_1.22.0 scuttle_1.4.0 celda_1.10.0 beepr_1.3
## [33] DropletUtils_1.14.2 SingleCellExperiment_1.16.0 SummarizedExperiment_1.24.0 Biobase_2.54.0
## [37] GenomicRanges_1.46.1 GenomeInfoDb_1.30.1 IRanges_2.28.0 S4Vectors_0.32.4
## [41] BiocGenerics_0.40.0 MatrixGenerics_1.6.0 matrixStats_0.62.0 ggplot2_3.3.6
## [45] plyr_1.8.7 sp_1.5-0 SeuratObject_4.1.1 Seurat_4.1.0.9007
##
## loaded via a namespace (and not attached):
## [1] rsvd_1.0.5 ica_1.0-3 corpcor_1.6.10 assertive.properties_0.0-4
## [5] foreach_1.5.2 lmtest_0.9-40 rprojroot_2.0.3 crayon_1.5.2
## [9] spatstat.core_2.4-4 rhdf5filters_1.6.0 backports_1.4.1 nlme_3.1-155
## [13] rlang_1.0.6 XVector_0.34.0 ROCR_1.0-11 irlba_2.3.5
## [17] SparseM_1.81 limma_3.50.3 xgboost_1.5.0.2 rjson_0.2.21
## [21] bit64_4.0.5 glue_1.6.2 sctransform_0.3.4 parallel_4.1.2
## [25] vipor_0.4.5 spatstat.sparse_2.1-1 AnnotationDbi_1.56.2 spatstat.geom_2.4-0
## [29] tidyselect_1.1.2 fitdistrplus_1.1-8 tidyr_1.2.1 assertive.types_0.0-3
## [33] zoo_1.8-10 org.Mm.eg.db_3.14.0 xtable_1.8-4 magrittr_2.0.3
## [37] evaluate_0.18 cli_3.4.0 zlibbioc_1.40.0 rstudioapi_0.13
## [41] miniUI_0.1.1.1 bslib_0.4.1 rpart_4.1.16 RcppEigen_0.3.3.9.2
## [45] shiny_1.7.3 BiocSingular_1.10.0 xfun_0.34 clue_0.3-60
## [49] cluster_2.1.2 tidygraph_1.2.1 KEGGREST_1.34.0 tibble_3.1.8
## [53] listenv_0.8.0 Biostrings_2.62.0 png_0.1-7 future_1.28.0
## [57] withr_2.5.0 bitops_1.0-7 ggforce_0.3.3 cellranger_1.1.0
## [61] assertive.base_0.0-9 dqrng_0.3.0 pillar_1.8.1 GlobalOptions_0.1.2
## [65] cachem_1.0.6 fs_1.5.2 GetoptLong_1.0.5 DelayedMatrixStats_1.16.0
## [69] vctrs_0.4.1 ellipsis_0.3.2 generics_0.1.3 tools_4.1.2
## [73] beeswarm_0.4.0 munsell_0.5.0 tweenr_1.0.2 DelayedArray_0.20.0
## [77] fastmap_1.1.0 compiler_4.1.2 pkgload_1.2.4 abind_1.4-5
## [81] httpuv_1.6.6 plotly_4.10.0 rgeos_0.5-9 GenomeInfoDbData_1.2.7
## [85] gridExtra_2.3 enrichR_3.0 edgeR_3.36.0 lattice_0.20-45
## [89] deldir_1.0-6 utf8_1.2.2 later_1.3.0 jsonlite_1.8.3
## [93] multipanelfigure_2.1.2 scales_1.2.1 graph_1.72.0 ScaledMatrix_1.2.0
## [97] pbapply_1.5-0 sparseMatrixStats_1.6.0 lazyeval_0.2.2 promises_1.2.0.1
## [101] doParallel_1.0.17 R.utils_2.12.0 goftest_1.2-3 checkmate_2.0.0
## [105] spatstat.utils_2.3-1 reticulate_1.26 textshaping_0.3.6 statmod_1.4.36
## [109] Rtsne_0.16 uwot_0.1.14 igraph_1.3.4 HDF5Array_1.22.1
## [113] survival_3.2-13 rsconnect_0.8.25 yaml_2.3.6 systemfonts_1.0.4
## [117] htmltools_0.5.3 memoise_2.0.1 locfit_1.5-9.6 graphlayouts_0.8.0
## [121] digest_0.6.30 assertthat_0.2.1 rappdirs_0.3.3 mime_0.12
## [125] RSQLite_2.2.17 future.apply_1.9.1 blob_1.2.3 R.oo_1.25.0
## [129] ragg_1.2.1 splines_4.1.2 labeling_0.4.2 Rhdf5lib_1.16.0
## [133] RCurl_1.98-1.8 assertive.numbers_0.0-2 hms_1.1.1 rhdf5_2.38.1
## [137] colorspace_2.0-3 ggbeeswarm_0.6.0 shape_1.4.6 assertive.files_0.0-2
## [141] sass_0.4.2 RANN_2.6.1 circlize_0.4.14 audio_0.1-10
## [145] fansi_1.0.3 tzdb_0.2.0 brio_1.1.3 parallelly_1.32.1
## [149] R6_2.5.1 grid_4.1.2 ggridges_0.5.3 lifecycle_1.0.3
## [153] formatR_1.12 bluster_1.4.0 curl_4.3.2 jquerylib_0.1.4
## [157] leiden_0.4.3 testthat_3.1.2 desc_1.4.0 RcppAnnoy_0.0.19
## [161] org.Hs.eg.db_3.14.0 iterators_1.0.14 stringr_1.4.1 topGO_2.46.0
## [165] htmlwidgets_1.5.4 beachmat_2.10.0 polyclip_1.10-0 purrr_0.3.4
## [169] gridGraphics_0.5-1 ComplexHeatmap_2.10.0 mgcv_1.8-38 globals_0.16.1
## [173] spatstat.random_2.2-0 progressr_0.11.0 codetools_0.2-18 GO.db_3.14.0
## [177] metapod_1.2.0 MCMCprecision_0.4.0 R.methodsS3_1.8.2 gtable_0.3.1
## [181] DBI_1.1.3 highr_0.9 tensor_1.5 httr_1.4.4
## [185] KernSmooth_2.23-20 vroom_1.5.7 stringi_1.7.8 reshape2_1.4.4
## [189] farver_2.1.1 combinat_0.0-8 BiocNeighbors_1.12.0 scattermore_0.8
## [193] bit_4.0.4 spatstat.data_2.2-0 pkgconfig_2.0.3 corrplot_0.92
## [197] knitr_1.40